Recombinant halohydrin dehalogenase, encoding gene, vector, engineering bacteria, and applications of recombinant halohydrin dehalogenase
A technology of halohydrin dehalogenase and coding gene, which is applied in the field of recombinant halohydrin dehalogenase, can solve the problems of low catalytic efficiency and poor thermal stability tolerance, and achieve the effect of high catalytic activity and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
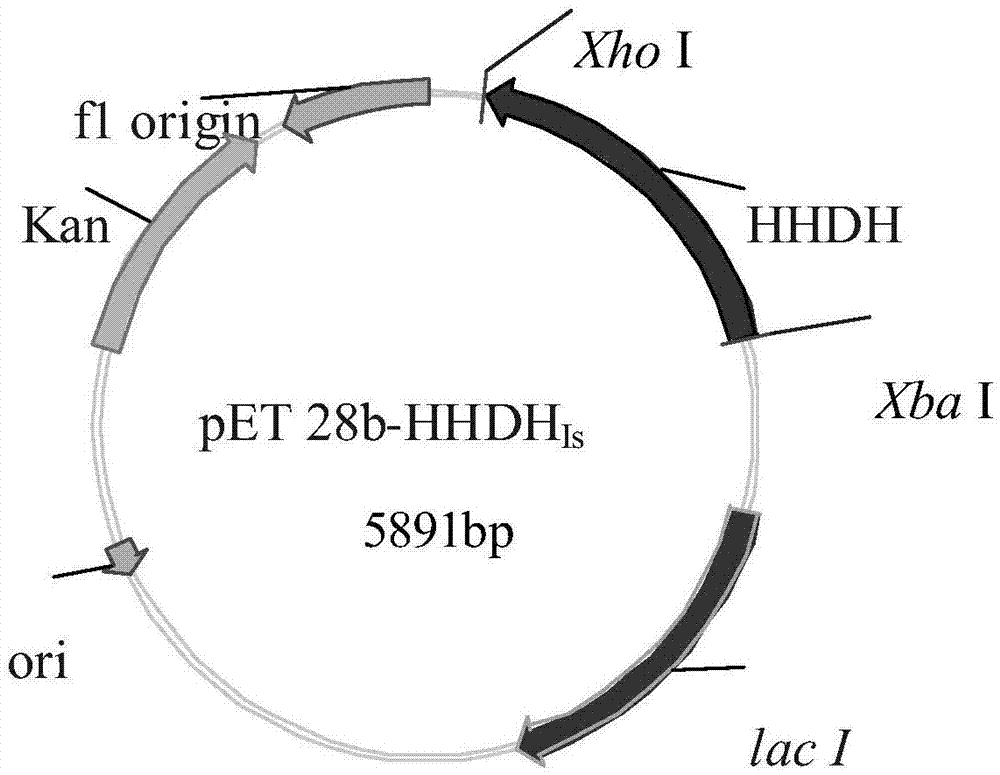
[0021] The acquisition of embodiment 1 parental gene and the preparation of recombinant expression plasmid and recombinant engineered bacteria
[0022] Using the halohydrin dehalogenase (Hhe B) reported in the literature as a probe, the homologous amino acid sequence was searched in the NCBI database, and it was found that the sequence was similar to the short-chain dehydrogenase predicted to be Idiomarina salinarum included in Genbank (Genbank No.KFZ32061. 1) The homology is 45%. Through sequence comparison, it is found that the short-chain dehydrogenase has the same catalytic triplet and some key conserved sequences as the halohydrin dehalogenase. It is speculated that the hydrolyzed protein is a suspected halohydrin dehalogenase enzyme. Because NCBI published the gene sequence of the short-chain dehydrogenase and the codon preference of heterologous expression in Escherichia coli, a synthetic gene was designed and delivered to Shanghai Xuguan Biotechnology Development Co., ...
Embodiment 2
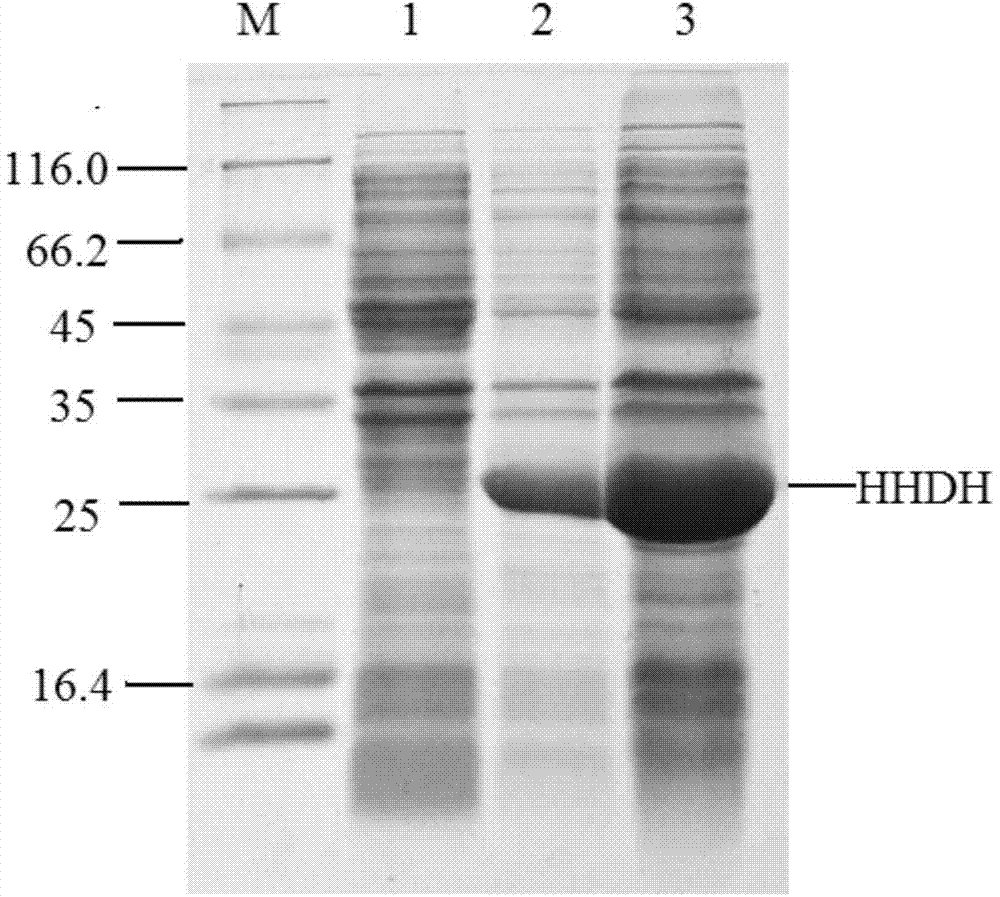
[0024] Embodiment 2 Expression of recombinant halohydrin dehalogenase
[0025] The recombinant Escherichia coli (E.coli) BL21(DE3) / pET28b-HHDH obtained in Example 1 Is , inoculated into LB medium containing a final concentration of 50 mg / L kanamycin sulfate, cultured with shaking at 37°C overnight, and inserted into a 500mL Erlenmeyer flask containing 100mL LB medium at an inoculum size of 1% (v / v) , placed on a shaker at 37°C and 180rpm for shaking culture, when the OD 600 of the culture medium reached 0.6, added IPTG with a final concentration of 0.5mM as an inducer, induced at 28°C for 10h, centrifuged the culture medium, and collected cells (that is, wet cells ), and washed twice with phosphate buffered saline to obtain resting cells. Suspend the obtained resting cells in 20 mL of phosphate buffer solution with pH 8.0, ultrasonically disrupt in an ice bath for 15 min (power 400W, break for 2 seconds and stop for 1 second), and collect the supernatant by centrifugation, wh...
Embodiment 3
[0026] Example 3: Synthesis of (R)-4-cyano-3-hydroxybutyrate ethyl from 50 g / L (S)-4-chloro-3-hydroxybutyrate catalyzed by halohydrin dehalogenase
[0027] With the recombinant Escherichia coli BL21(DE3) / pET28b-HHDH containing the intracellular expression recombinant plasmid obtained in Example 2 Is Wet cells were used as transformation enzymes (E.coli.BL21(DE3), E.coli.BL21(DE3) / pET28b and uninduced E.coli.BL21(DE3) / pET28b-HHDH Is As a control), with (S)-4-chloro-3-hydroxybutyric acid ethyl ester as a substrate, the conversion reaction was carried out to prepare (R)-4-cyano-3-hydroxybutyric acid ethyl ester. The composition of the transformation system and the transformation operation are as follows: add 30mL of 100mM phosphate buffer (pH 7.5) to a 100mL flask, add wet bacteria to make the concentration of the bacteria 10g / L buffer, and use a constant pH / potentiometric titrator to add NaCN to adjust When the pH reached 7.5, (S)-4-chloro-3-hydroxybutyric acid ethyl ester with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com