Acetagastrodin derivative, preparation method, preparation, and applications thereof
A technology for acetylgastrodin and its derivatives, which is applied in the field of acetylgastrodin derivatives and its preparation, can solve the problems of delayed bleeding risk and high requirements for purification technology, and achieve simple and easy preparation methods, good reproducibility, and selectivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
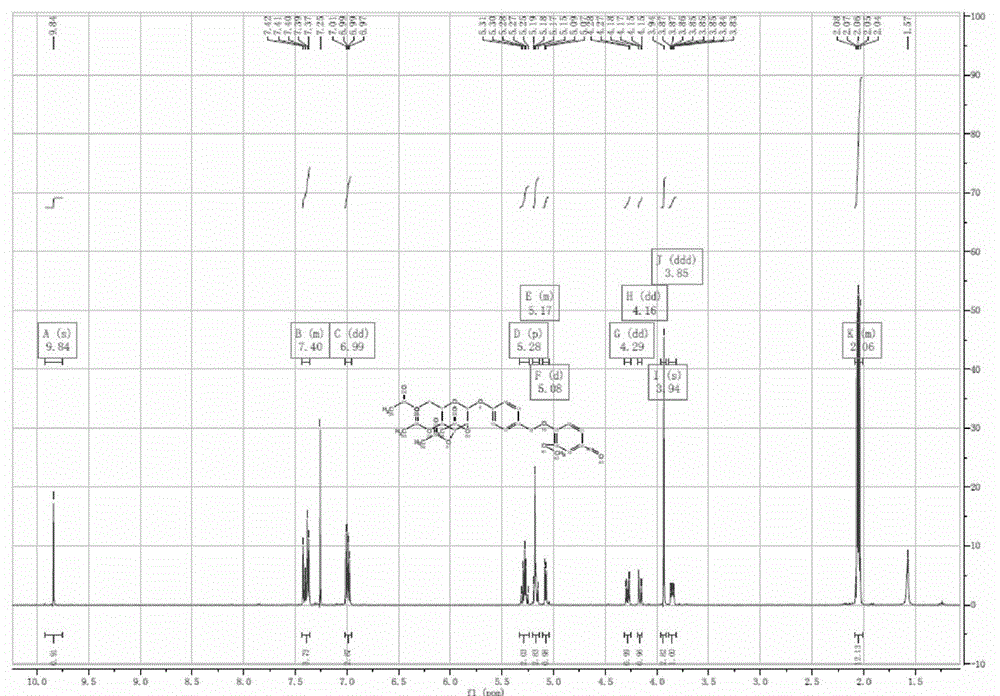
[0032] The preparation method of the acetylgastrodin derivatives of the present invention is based on ethyl p-hydroxybenzoate, p-hydroxybenzaldehyde, vanillin, nicotinic acid, triacetyl gallic acid, cinnamic acid, acetylsalicylic acid, butanediol Acid anhydride or potassium 4-hydroxybutyrate is used as the raw material to undergo condensation reaction with acetylgastrodin to obtain the target product.
[0033]
[0034]
[0035] Among them, R - for
[0036] , , , , , , , or
[0037] Described condensation reaction specifically comprises:
[0038] the first sort:
[0039] Step 1, dissolve the compound shown in formula II in an organic solvent, add thionyl chloride dropwise under stirring in an ice bath, stir and react to obtain the compound shown in formula III, add an organic solvent for later use;
[0040] Step 2, ethyl p-hydroxybenzoate, p-hydroxybenzaldehyde, potassium 4-hydroxybutyrate, and vanillin are heated with the compound shown in formula...
Embodiment 1
[0069] Preparation of acetylgastrodin p-hydroxybenzaldehyde etherate:
[0070] a. Preparation of intermediate acetylgastrodin benzyl chloride
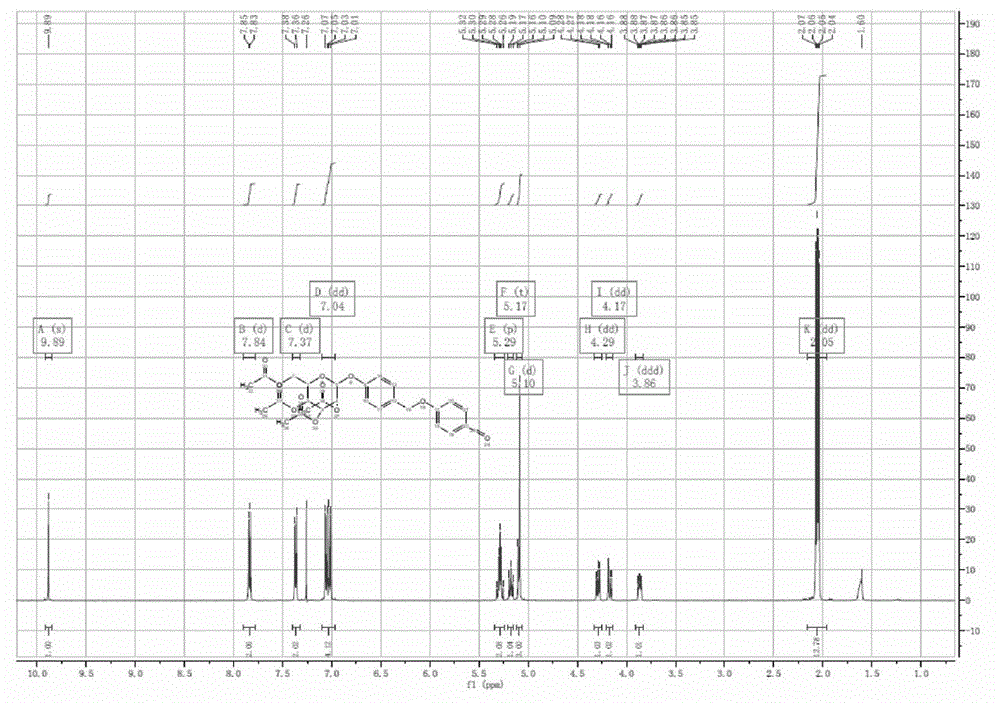
[0071] In the reaction flask, add acetylgastrodin (4.55g, 0.01mol), dichloromethane 20ml, and add thionyl chloride (2ml, 0.028mol) dropwise under stirring in an ice bath. React at 0-25°C for 2 hours, distill off the solvent and the remaining thionyl chloride under reduced pressure to obtain 4.8 g of white solid with a yield of 98%.
[0072] b. Preparation of acetylgastrodin p-hydroxybenzaldehyde etherate
[0073] In the reaction flask, add p-hydroxybenzaldehyde (1.22g, 0.01mol), 20ml of acetone, and potassium carbonate (3g, 0.02mol). Stir at 50°C for 30min, add dropwise an acetone solution of acetylgastrodin benzyl chloride (4.8g, 0.01mol), and react at 70°C for 5h. Potassium carbonate was removed by filtration, and the filtrate was concentrated under reduced pressure. Add 50 ml of dichloromethane, wash with water and saturated bri...
Embodiment 2
[0077] Preparation of acetylgastrodin ethyl paraben etherate:
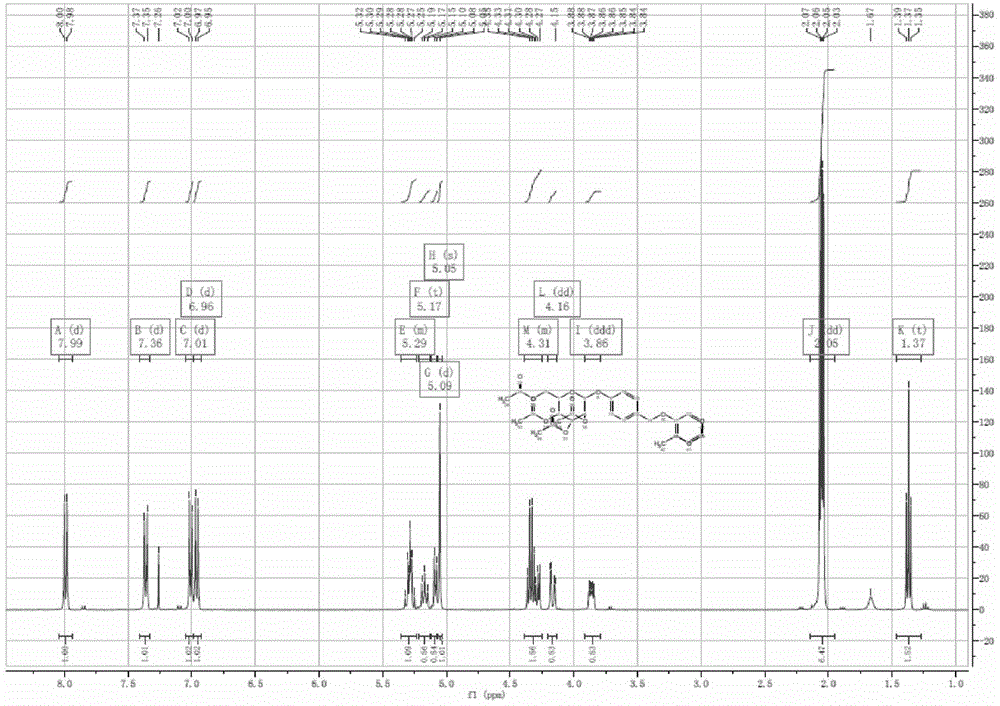
[0078] Preparation of the compound acetylgastrodin ethylparaben etherate of the present invention Referring to Example 1, 4 g of acetylgastrodin ethylparaben etherate was prepared as a white solid with a yield of 66%.
[0079] The compound NMR identification is as follows:
[0080] 1 H-NMR (500MHz, CDCl 3 , δppm): δ7.99 (d, 2H), δ7.36 (d, 2H), δ7.01 (d, 2H), δ6.96 (d, 2H), δ5.29 (m, 2H), δ5 .17(t, 1H), δ5.09(d, 1H), δ5.05(s, 2H), δ4.31(m, 3H), δ4.16(d, 1H), δ3.86(m, 1H), δ2.05 (m, 12H), δ1.37 (t, 3H). The structural formula is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com