Imatinib immunogen, derivative, synthesis method, specific antibody and detection reagent and preparation methods
A technology of imatinib and its derivatives, which is applied in biological testing, immunoglobulin, biomaterial analysis, etc., can solve the problems that are not suitable for large-scale clinical application and cannot meet the needs of clinical testing, so as to reduce testing costs and combine Powerful, accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis and Quantitative Detection of Embodiment 1 Imatinib Derivatives
[0068] The chemical structure of imatinib derivative is shown in formula (IV):
[0069]
[0070] The synthetic route and preparation steps of the above-mentioned imatinib derivatives are as follows:
[0071]
[0072] Concrete synthetic steps are as follows:
[0073] Synthesis of compound 3
[0074]
[0075] Weigh 20g (87.31mmol) of compound 1, dissolve in CH 3 CN, then weigh 18g (130.96mmol) K 2 CO 3 and 19.5g (104.77mmol) of compound 2 (1-tert-butoxycarbonylpiperazine) were sequentially added to the above solution to prepare a reaction mixture solution. After the addition of all ingredients was complete, the above reaction mixture was stirred overnight at 60°C. The solvent was evaporated by a reduced pressure method, and then the obtained residue was dissolved in 300mL of EA, washed with 60mL of brine, and finally the organic phase was passed through Na 2 SO 4 After drying and ...
Embodiment 2
[0095] The synthesis of embodiment two imatinib immunogens
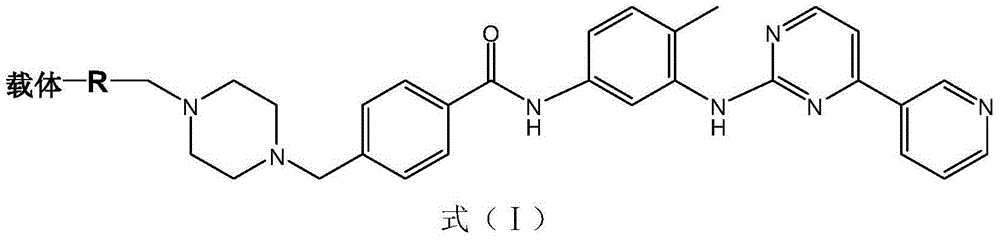
[0096] The imatinib immunogen is composed of bovine serum albumin (Bovine Serum Albumin, BSA) and imatinib derivatives represented by formula (II) -(CH 2 ) n The -COO- group is connected. In this embodiment, the synthesis method of the immunogen is described in detail by taking n=4 as an example. The specific steps are as follows:
[0097] 1. Dissolve 200mg bovine serum albumin in 50ml 0.2M, pH 8.5 phosphate buffer;
[0098] 2. Add the following chemicals into a small beaker and stir to dissolve: 200mg of synthetic imatinib derivatives, 3.5ml of dimethylformamide, 3.5ml of ethanol, 7.0ml of 10mM potassium phosphate buffer at pH 5.0, 200mg of 1 -Ethyl-3-(-3-dimethylaminopropyl)carbodiimide, 50mg N-hydroxysulfosuccinimide, these chemicals were stirred and dissolved at room temperature for 30min;
[0099] 3. The dissolved solution was added dropwise to the BSA solution, and stirred overnight at 2-8°C to obtain the an...
Embodiment 3
[0100] Example 3: Preparation of Anti-Imatinib Specific Antibody
[0101] The imatinib immunogen prepared in Example 2 was inoculated into experimental animal rabbits by conventional methods, and the antiserum was taken after booster immunization. The specific steps were as follows:
[0102] 1. Dilute the above synthesized imatinib immunogen to 1.0 mg / ml with PBS to obtain an antigen solution, then mix 1.0 ml of the antigen solution with Freund's complete adjuvant, and inject the experimental animal rabbit.
[0103] 2. After 2-3 weeks, inject 1.0ml of the same antigen solution and incomplete Freund's adjuvant to the above-mentioned experimental animal rabbit once, and then inject once every four weeks, a total of 4 injections.
[0104] 3. Take blood from the experimental animal rabbit in step 2, separate and purify to obtain anti-imatinib specific antibody with a titer of 1:30000-1:50000.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com