Ranitidine hydrochloride freeze-dried powder injection compound for treatment of digestive system diseases
A technology for ranitidine hydrochloride and digestive system diseases, applied in the field of medicine, can solve the problems of tissue hypoxia, high production cost of freeze-dried powder injection, poor quality of finished products, etc., and achieve small changes in the number of insoluble particles and a small number of insoluble particles , Solve the effect of extremely deliquescent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
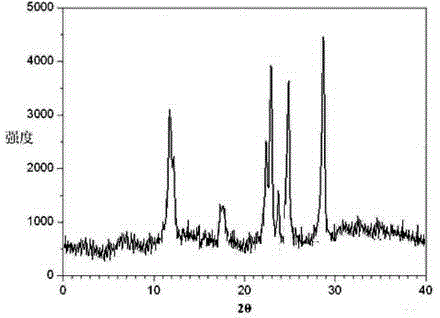
[0027] Example 1: Preparation of Ranitidine Hydrochloride Crystal
[0028] (1) Add the crude ranitidine hydrochloride to a mixed solution of methanol, carbon tetrachloride and dichloromethane whose volume is 7 times the weight of ranitidine hydrochloride. The volume ratio is 4.5:2.5:1, the temperature is raised to 35°C, and it is stirred until it is completely dissolved;
[0029] (2) Under a sound field with a frequency of 25KHz and an output power of 45W, add a mixed solution of acetone, ethanol and chloroform with a volume of 8 times the weight of ranitidine hydrochloride while stirring. The volume ratio of acetone, ethanol and chloroform is 3.5 :1:1, the stirring speed is 250 rpm;
[0030] (3) After adding the mixed solution of acetone, ethanol and chloroform, in a sound field with a frequency of 30KHz and an output power of 20W, the temperature is reduced to -2°C at 12°C / hour, the crystals are grown for 3 hours, washed, and vacuum dried to obtain Ranitidine hydrochloride compo...
Embodiment 2
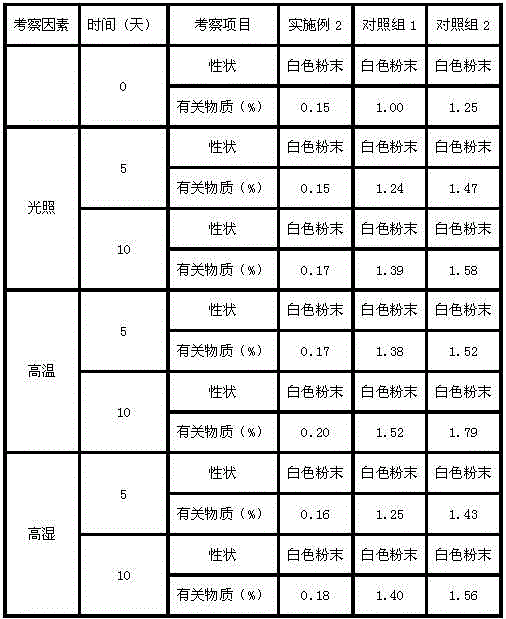
[0032] Example 2: Preparation of Ranitidine Hydrochloride Lyophilized Powder Injection:
[0033] Prescription: in parts by weight: 1 part of the ranitidine hydrochloride crystal compound prepared in Example 1 and 1.6 parts of low molecular dextran.
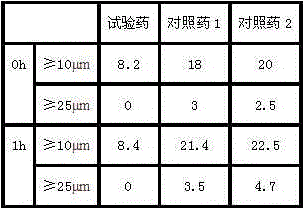
[0034] Take the ranitidine hydrochloride compound of the present invention, stir and dissolve it with water for injection, add a prescription amount of low molecular dextran, adjust the pH to 5.5-6.5, and then stir until the pH does not change, then add water for injection until the volume of the solution is hydrochloric acid 100 times the weight of ranitidine, then coarsely filter with activated carbon, sterilize and filter through 1.0μm, 0.45μm, 0.22μm microporous membranes in sequence, filter into a sterile room, measure the pH and content and then fill it, Press a half plug, put it in a freeze-drying box that has been cooled to -45°C, freeze-dry at low temperature, press the plug out of the box, and roll the lid.
[0035] The freez...
Embodiment 3
[0036] Example 3: Preparation of Ranitidine Hydrochloride Lyophilized Powder Injection:
[0037] Prescription: in parts by weight: 1 part of the ranitidine hydrochloride crystal compound prepared in Example 1 and 0.8 parts of low molecular dextran.
[0038] Take the ranitidine hydrochloride compound of the present invention, stir and dissolve it with water for injection, add a prescription amount of low molecular dextran, adjust the pH to 5.5-6.5, and then stir until the pH does not change, then add water for injection until the volume of the solution is hydrochloric acid 100 times the weight of ranitidine, then coarsely filter with activated carbon, sterilize and filter through 1.0μm, 0.45μm, 0.22μm microporous membranes in sequence, filter into a sterile room, measure the pH and content and then fill it, Press a half plug, put it in a freeze-drying box that has been cooled to -45°C, freeze-dry at low temperature, press the plug out of the box, and roll the lid.
[0039] The freez...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com