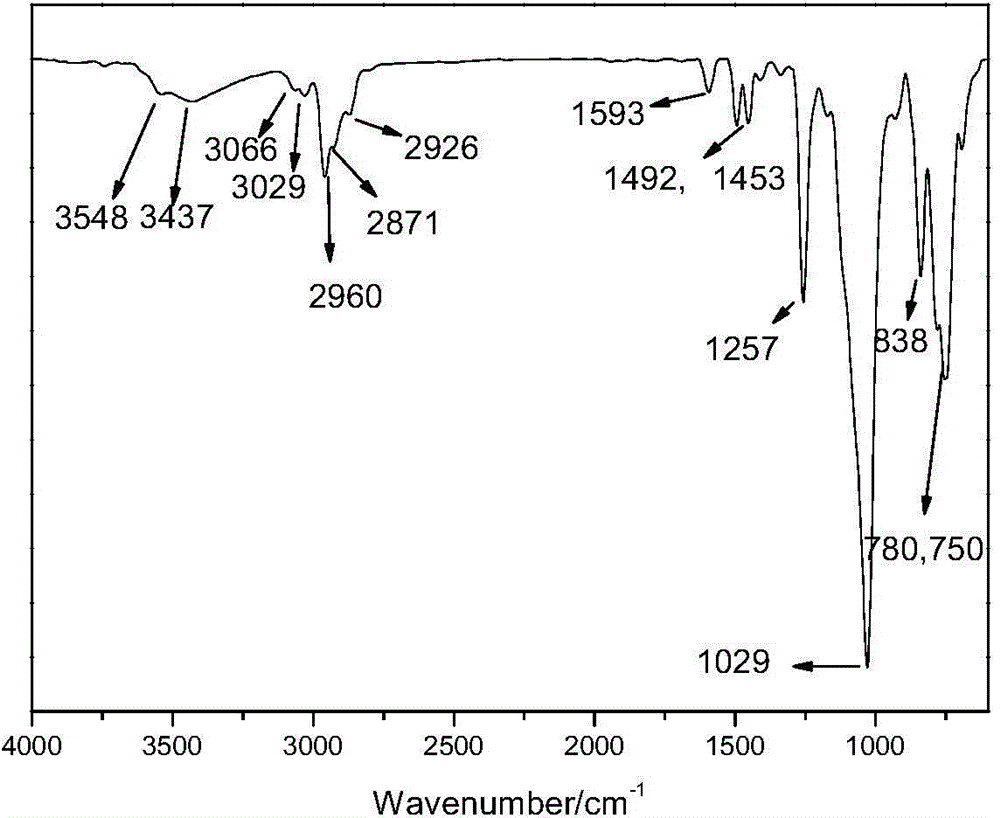
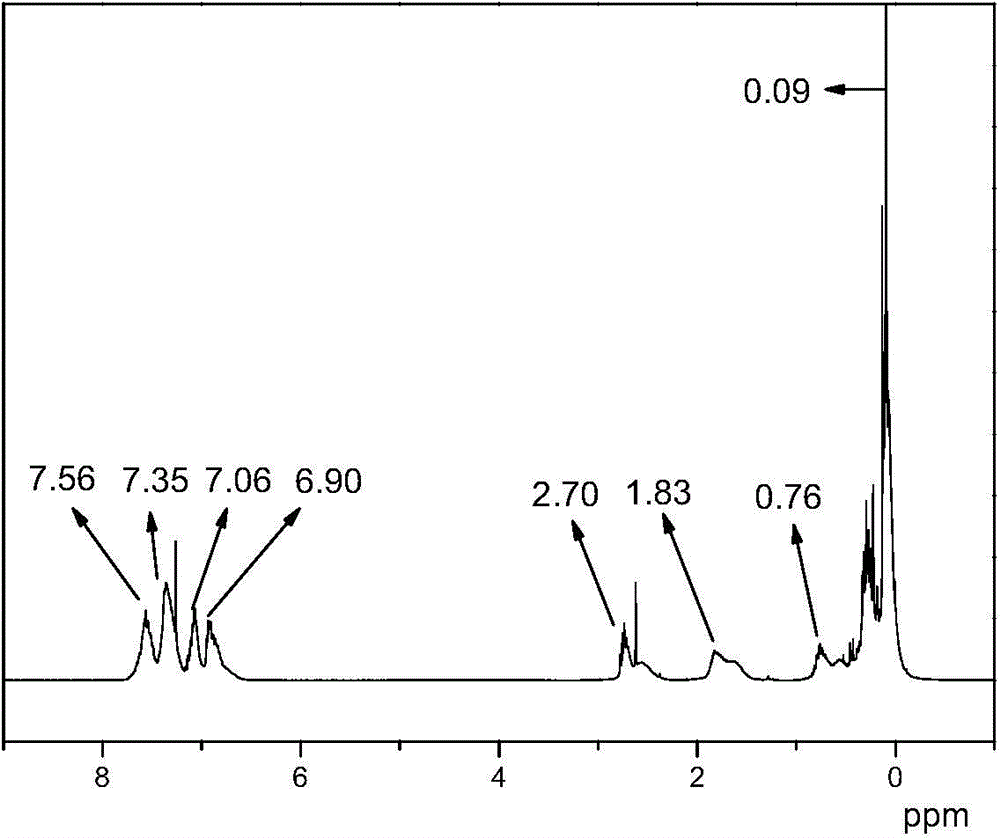
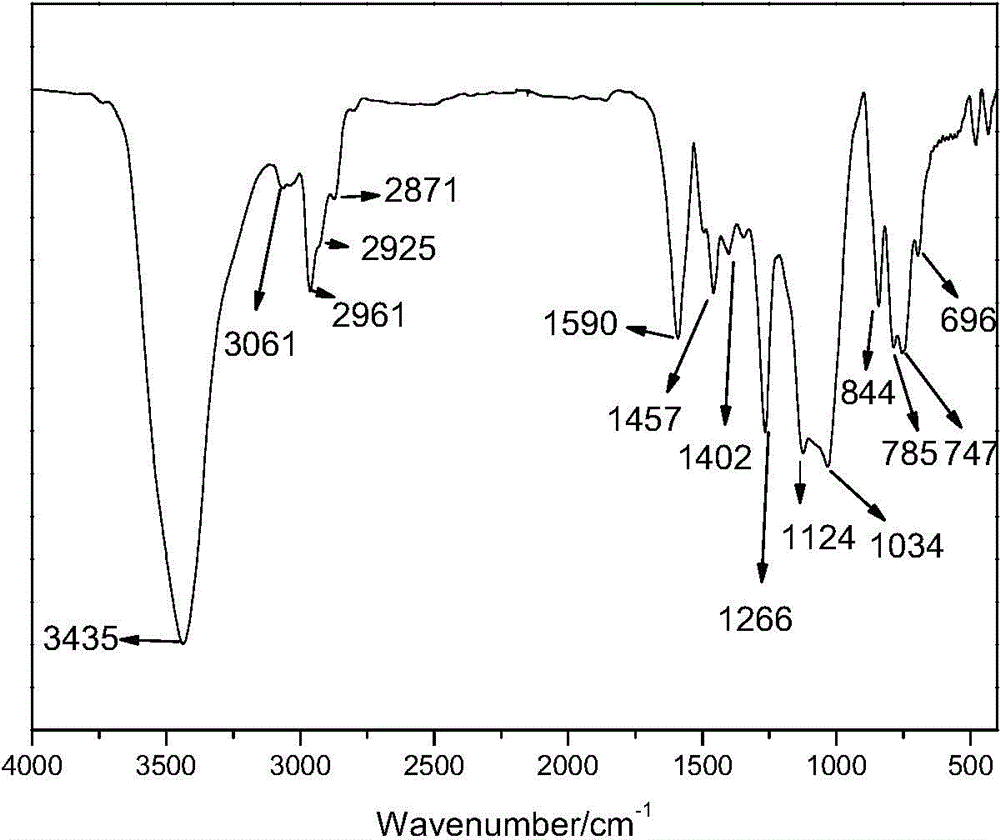
Organosilicon high-molecular material and preparation method thereof
A silicon polymer and organosilicon technology, applied in the field of organosilicon synthesis, can solve problems such as poor compatibility, intolerance to hydrolysis, and separation of microstructures, and achieve the effect of good elasticity and controllable hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1, the preparation of the modified polysiloxane that side chain contains phenol group
[0056] In a use N 2 In the replaced container, add 32.1g of 2-allylphenyl trimethylsilyl ether, 11.5g of methyldichlorosilane, 40mL of toluene as solvent, 0.1g of Karstedt catalyst containing Pt complex, and react at 80°C Until the Si-H bond completely disappears, the solvent toluene is removed to obtain the hydrosilylation product γ-phenyltrimethylsilyl ether methyl dichlorosilane. React 53.5 g of the hydrosilylation products γ-phenyltrimethylsilyl ether methyl dichlorosilane and 33.33 g of methylphenyldialkoxysilane in sufficient water, and adjust the pH to 3 with hydrochloric acid before the reaction. After liquid separation to remove small molecules, add 2.7 g of hexamethyldisiloxane and 0.26 g of trifluoromethanesulfonic acid, react for 3 hours, remove small molecules after neutralizing the catalyst, and obtain a modified compound with a phenol group-containing chain...
Embodiment 2
[0057] Embodiment 2, the preparation of the modified polysiloxane that side chain contains phenol group
[0058] In a use N 2 In the replaced container, add 32.1g of 2-allylphenyl trimethylsilyl ether, 17.7g of phenyldichlorosilane, 40mL of toluene as solvent, 0.1g of Karstedt catalyst containing Pt complex, and react at 80°C Until the Si-H bond completely disappears, the solvent toluene is removed to obtain the hydrosilylation product γ-phenyltrimethylsilyl ether phenyldichlorosilane. React 63.5 g of the hydrosilylation products γ-phenyltrimethylsilyl ether phenyldichlorosilane and 33.33 g of methylphenyldialkoxysilane in sufficient water, and adjust the pH to 3 with hydrochloric acid before the reaction. Add 2.7 g of hexamethyldisiloxane and 0.26 g of trifluoromethanesulfonic acid after liquid separation to remove small molecules, react for 3 hours, remove small molecules after neutralizing the catalyst, and obtain a modified compound with a phenol group-containing chain se...
Embodiment 3
[0059] Embodiment 3, the preparation of the modified polysiloxane that side chain contains phenol group
[0060] As in Example 1, 21.4 g of the hydrosilylation product γ-phenyltrimethylsilyl ether methyl dichlorosilane and 48.53 g of methylphenyldialkoxysilane were reacted in a sufficient amount of water, and hydrochloric acid was used before the reaction. Adjust pH=3. After liquid separation to remove small molecules, add 2.7 g of hexamethyldisiloxane and 0.26 g of trifluoromethanesulfonic acid, react for 3 hours, remove small molecules after neutralizing the catalyst, and obtain a modified compound with a phenol group-containing chain segment ratio of 20%. polysiloxane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com