Construction and expression method of recombinant G-CSF (15-75) polypeptide vector
A GS115 and carrier technology, applied in the field of biopharmaceuticals, can solve the problems of easy gene loss, low product expression, low renaturation rate, etc., and achieve the effect of easy purification, simple purification method and short fermentation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
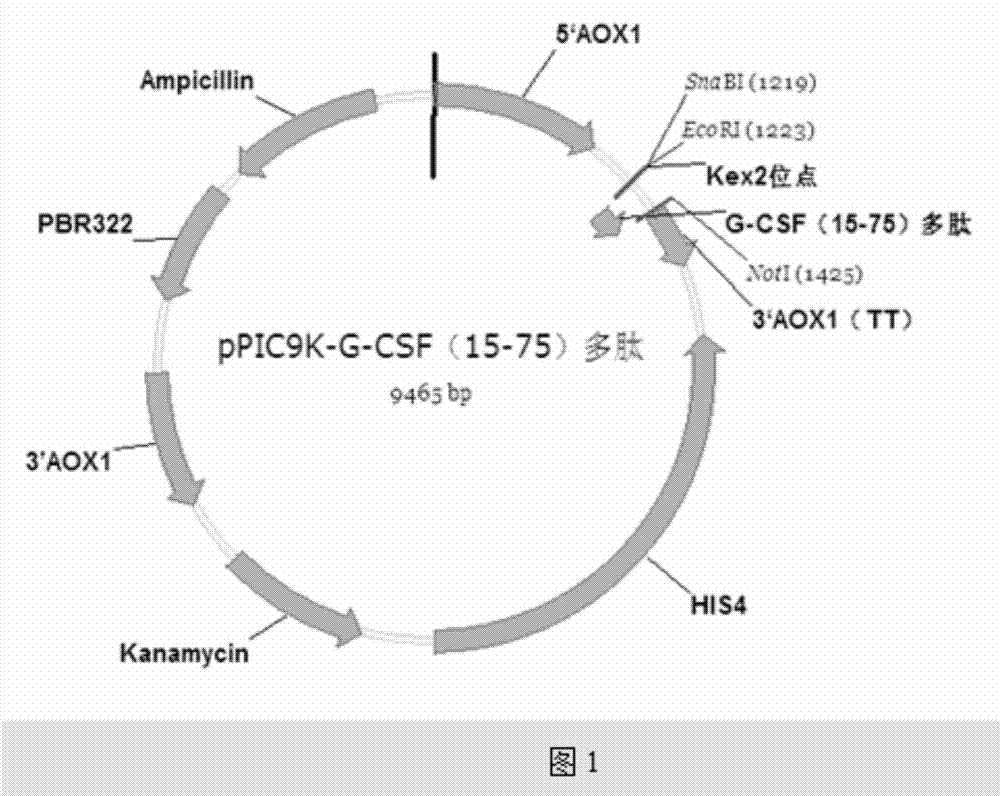
[0037] Embodiment 1, recombinant plasmid construction
[0038] 1. Gene Design
[0039]The base sequence of the 15th to 75th amino acids of the unoptimized G-CSF sequence is:
[0040] CT AAGTG TT GA CAAGT G AAGAT CA GG GATGG GC GC CT CA GA AAA CT TGTGC AC TACAAG TGTG CACCC GA GA TGGT CTGCT GG CAC TCTGGG ATCCC TGGGCTCC CTGAG AG TGCCC AG CA GC CTGCA TGGCAGG TG TTG.
[0041] According to the full human G-CSF sequence, the G-CSF gene from the 15th to the 75th amino acid was optimized by using common yeast codons (marked in bold black), and an EcoR Ⅰ site and kex2 enzyme were added at the 5′ end Cutting site, TAA stop codon and Not I site are added to the 3′ end, as shown in the underlined part, the full-length sequence of the synthesized gene is:
[0042] 5' -GAATTCGAAAAACGC CT AAGTG TT GA CAAGT G AAGAT CA GG GATGG GC GC CT CA GA AAA CT TGTGC AC TACAAG TGTG ...
Embodiment 2
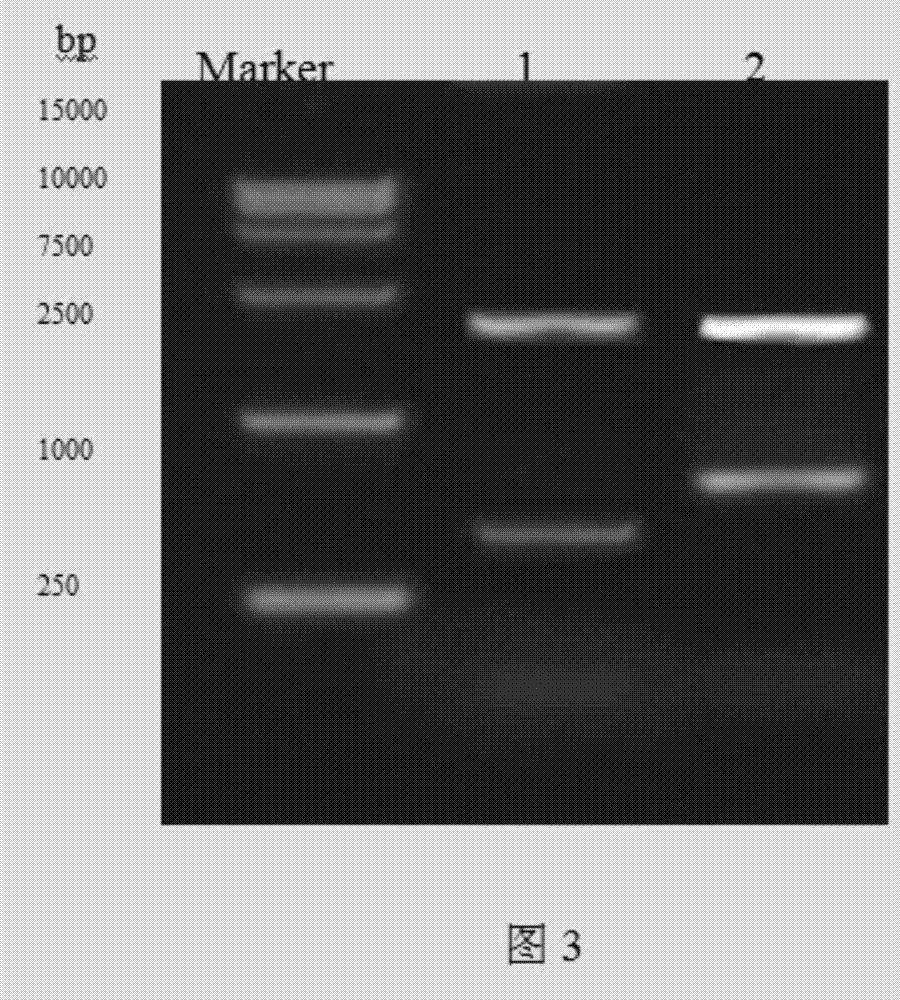
[0073] Example 2. Electroporation of recombinant plasmids integrated into Pichia pastoris cells
[0074] 1. Preparation of Pichia Competent Cells:
[0075] 1. Pick a single colony of yeast GS115 and inoculate it into a 5ml YPD test tube, incubate at 200rpm at 30°C for 6 hours, then transfer it to a 50ml YPD shaker flask at 1% inoculum size, and incubate overnight at 200rpm at 30°C;
[0076] 2. Pre-cool the centrifuge tube for standby, pre-cool the bacteria solution at 4°C for 30 minutes, and collect the bacteria by centrifugation at 5000 rpm for 10 minutes;
[0077] 3. Add 40ml 4℃ pre-cooled sterilized water to wash twice;
[0078] 4. Add 20ml of 1mol / L sorbitol pre-cooled at 4℃ and wash once;
[0079] 5. Add 500 μL of 1mol / L sorbitol pre-cooled at 4°C to the mixed cells, and aliquot 80 μL / cartridge.
[0080] 2. Plasmid linearization:
[0081] 1. Plasmid linearization enzyme digestion:
[0082]
[0083] Digest overnight at 37°C.
[0084] 2. Heat up the digested sample...
Embodiment 3
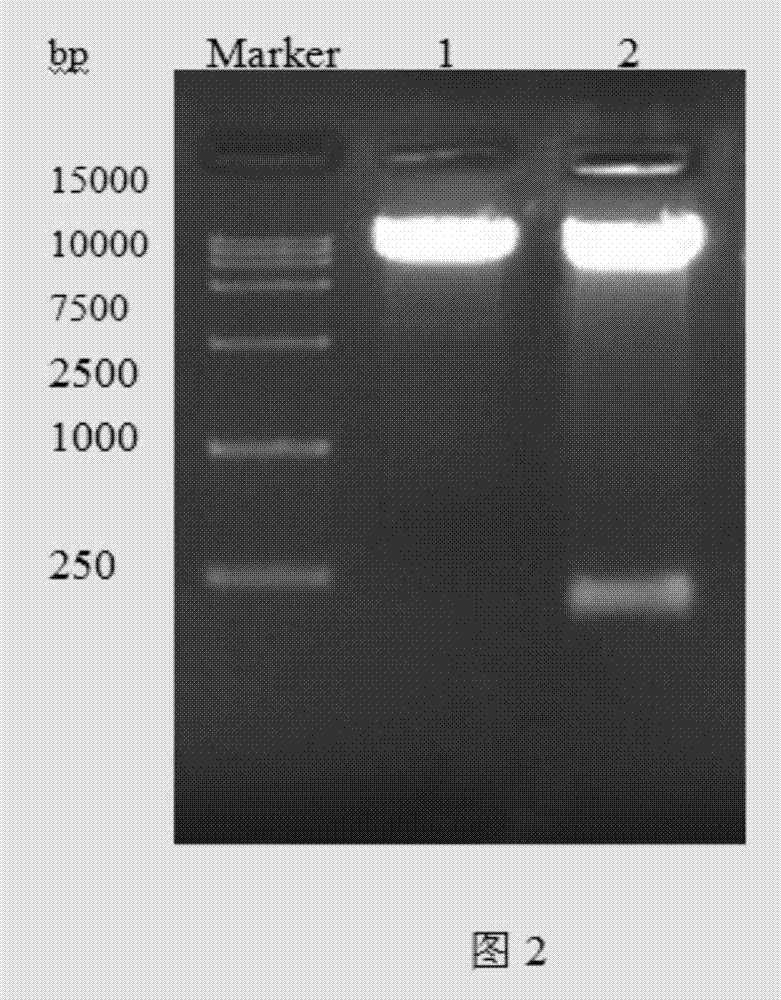
[0105] Embodiment 3: G-CSF (15-75) strain shake flask expression
[0106] In order to verify the expression level of recombinant G-CSF (15-75) polypeptide, Pichia pastoris (pPIC9K-G-CSF(15-75)-GS115) (A1-A5), Pichia pastoris (pPIC9K-G- CSF-GS115)(B1-B5) and negative control strain GS115(C) were used for preliminary expression studies on shake flasks;
[0107] 1. Inoculate each bacterial strain in BMGY medium (1% yeast powder, 2% peptone, 100mM potassium phosphate pH6.0, 1.34% YNB4×10 -5 % biotin, 1% glycerol), inoculate three bottles per plant, culture at 30°C for 24h, OD 600 reach 4;
[0108] 2. Centrifuge the bacterial solution at 1200rpm for 30min to collect the cells, remove the supernatant, and use BMMY medium (1% yeast powder, 2% peptone, 100mM potassium phosphate pH6.0, 1.34%YNB4×10 -5 % biotin, 0.5% methanol) to resuspend the bacteria, induce expression, and induce 80h to end the culture;
[0109] 3. After centrifuging the fermentation broth at 12000 rpm for 10 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com