Tetanus toxoid resisting monoclonal antibody, hybridoma cell strain for producing antibody, and application of antibody
A tetanus toxoid, hybridoma cell line technology, applied in antibacterial immunoglobulins, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of quality control uncertainty, interference with test results, etc. The effect of improved sensitivity, simple operation and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of hybridoma cell line WV-TT-05
[0021] 1.1 Source of antigen
[0022] Tetanus toxoid antigen standard (TT standard) was purchased from China National Institutes for Food and Drug Control, and the other three batches of tetanus toxoid were prepared according to Part Three of the Pharmacopoeia of the People's Republic of China 2010 Edition.
[0023] 1.2 Source of mice
[0024] Female, 6-week-old BALB / c mice, SPF (Specific Pathogen Free, SPF) grade, 12-15 g, were purchased from the Experimental Animal Center of Kunming Medical University (permit number: SCXK Dian 2011-0004).
[0025] 1.3 Source of myeloma cells
[0026] Myeloma cells SP2 / 0 were purchased from the Kunming Cell Bank (KCB92021YJ) of the Type Culture Collection Center of the Chinese Academy of Sciences.
[0027] 1.4 Preparation of hybridoma cell lines
[0028] Monoclonal cells were prepared by in vivo immunization and polyethylene glycol (PEG) fusion.
[0029] 1.4.1 Mouse i...
Embodiment 2
[0051] Embodiment 2 hybridoma cell culture and the preparation of monoclonal antibody
[0052] 2.1 Paraffin injection
[0053] Two weeks before cell culture, take 3 BALB / c mice from 8 to 10 weeks old and inject paraffin, 0.5ml / mouse, to stimulate immune cells, cause granulocytes and macrophages to infiltrate inflammation, and produce a series of growth factors to facilitate the proliferation of hybridoma cells and the massive secretion of antibodies. Separate the cages, write the marks, and prepare to inject the hybridoma cells ten days later.
[0054] 2.2 Recovery and expansion of hybridoma cells
[0055] Take out the frozen cell tube from the liquid nitrogen tank, and quickly thaw the frozen hybridoma cells in a 37°C water bath. Put the thawed cells into the centrifuge, 800rpm, 8min, and centrifuge. Discard the supernatant, absorb 3ml of cell culture medium, mix well to precipitate the cells, aspirate the cell solution, mix well and inhale into a 6-well cell culture plat...
Embodiment 3
[0060] Embodiment 3 Monoclonal antibody titer determination of the present invention
[0061] 3.1 Ascites monoclonal antibody titer detection
[0062] 3.1.1 Indirect ELISA procedure
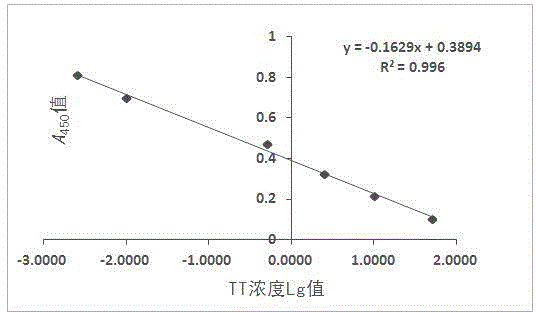
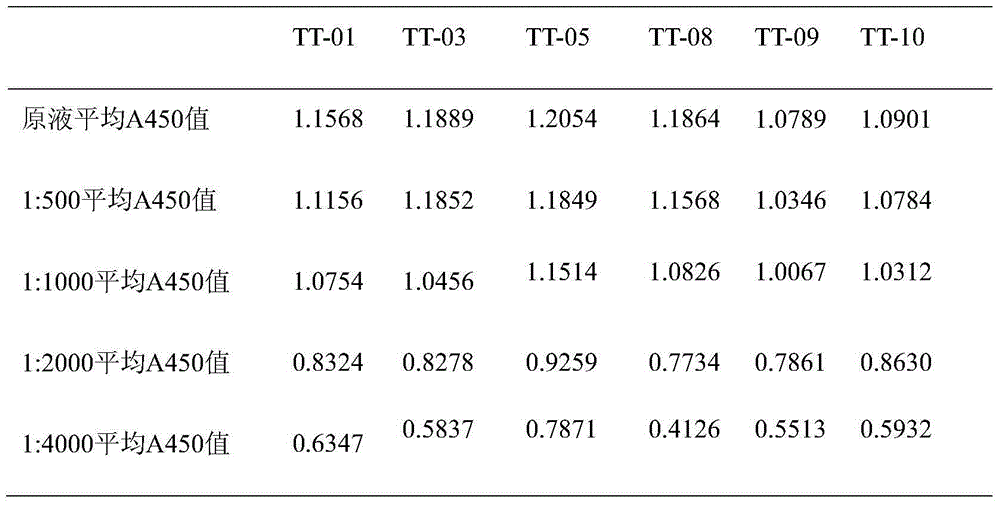
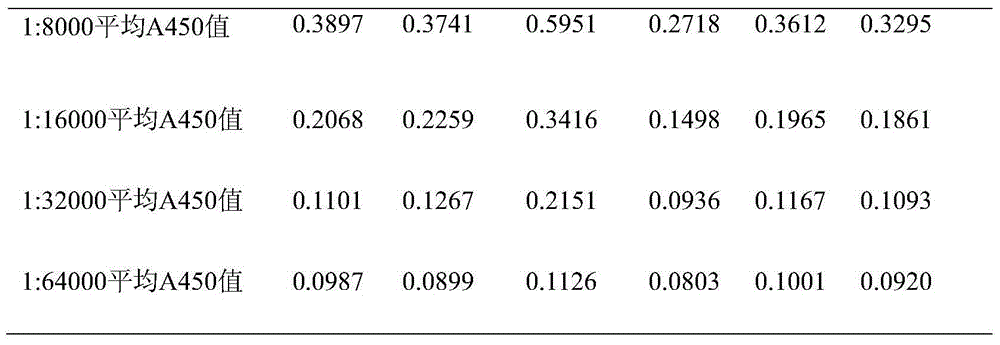
[0063] Dilute the TT standard to 0.5 μg / ml with the coating solution, coat the microtiter plate with 100 μl / well, and overnight at 4°C. Wash 3 times with washing solution the next day, block with 1% (volume ratio) bovine serum albumin in a 37°C incubator, wash 3 times with washing solution after 2 hours, and ascites with 0.01mol / l, pH 7.4 Dilute PBS according to the original times, 1:10, 1:100, 1:1000, 1:10000, 1:100000, 1:1000000, add 100 μl to each well, incubate in a 37°C incubator for 1 hour, wash the plate three times, add Horseradish peroxidase-labeled goat anti-mouse IgG enzyme-labeled secondary antibody (1:10000) diluted with 0.01mol / L, pH 7.4 PBS 100μl, incubated in a 37°C incubator for 1h, washed the plate 3 times, added 100μl substrate solution, measure A after color development 45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com