Thrombolytic enzyme gene, recombinant expression vector and recombinant bacteria comprising same and application
A recombinant carrier and thrombolytic enzyme technology, applied in the biological field, can solve the problems of low output of thrombolytic enzyme, high production cost, low enzyme activity, etc., and achieve the effects of reducing cost, increasing solubility and activity, and good practicability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation process of embodiment 1 Bacillus subtilis QK-02
[0034] Put the rice leaves at 85°C for heat treatment for 10 minutes, then wrap the sterilized soybeans with them, cultivate them at 42°C for 24 hours, and then soak the treated soybeans with the culture solution, and use the soaked soybean culture solution for 10 -2 ~10 -6Diluted, spread on nutrient agar plate, and cultured upside down at 37°C. The obtained colonies were respectively inoculated into 5 mL of culture solution, cultured at 37° C. at 250 rpm / min for 24 hours, and then centrifuged at 6000 rpm / min for 5 minutes. Take 20 μl of the supernatant and measure the thrombolytic enzyme activity on the fibrin plate, and then select the strain with the highest enzyme activity.
Embodiment 2
[0035] Embodiment 2 PCR amplification and sequence optimization of gene qk encoding thrombolytic enzyme
[0036] 2.1 PCR amplification and cloning of gene qk encoding Bacillus subtilis thrombolyticase
[0037] Genomic DNA extraction of Bacillus subtilis QK-02 was performed according to a conventional method (Saito, H. et al, 1963, Biochim. Biophys. Acta 72:619-629).
[0038] Primer design for PCR amplification:
[0039] Primer P1: 5'ACGCGTCGACATGGCGCAATCTGTTCCTTACGGC
[0040] Primer P2: 3'CCGGAATTCTAGCTATTATTTGTGCAGCTGC
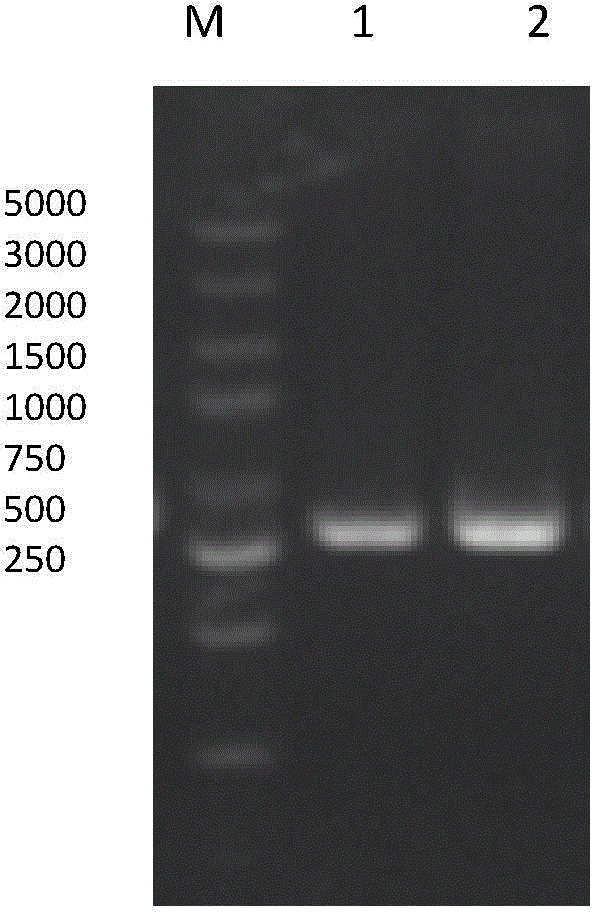
[0041] PCR amplification reaction system 50ul system: 5μl Bacillus subtilis genomic DNA as a template, 1μl each of primers P1 and P2 (10μM), 5μl of 10×PCR buffer, 5μl of dNTP (2mmol), 0.5μl of KOD enzyme, and finally use sterile water Make up to 50ul. The reaction program is: 94°C, 5min; 94°C, 30s; 56°C, 10s; 74°C, 30s; 74°C, 6min, a total of 35 cycles. After the amplification was completed, the PCR product was detected by 1% agarose gel electrophoresis,...
Embodiment 3
[0048] Embodiment 3 Construction of Thrombolytic Enzyme Recombinant Expression Vector
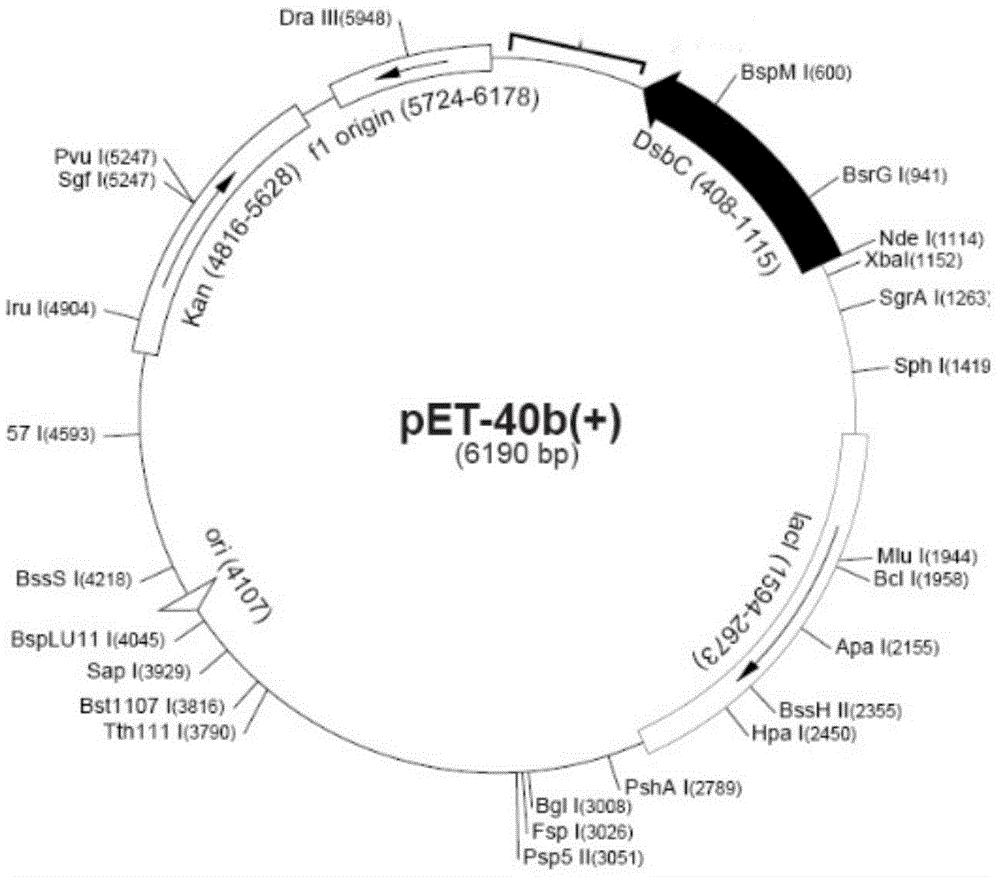
[0049] The optimized qk gene fragment and pET40b vector (see figure 2 ) were both digested by Sal I and EcoR I, and the fragments were recovered by gel and ligated with the carrier at 16°C overnight, and then passed through CaCl 2 Transformed into DH5α cells by the method, spread the transformed product on LB agar plate containing kanamycin (Kan) resistance, and incubate upside down at 37°C for 14-16h. After the positive clones were screened out, the plasmids were extracted for double-enzyme digestion identification, such as image 3 (M: Marker, 1: vector fragment before digestion, 2: vector after SalI / EcoRI double digestion), and the positive recombinants were sent to the company for sequencing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com