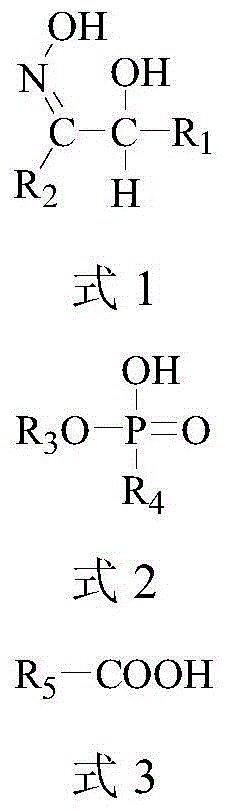Method for selectively extracting and recycling gallium, germanium and indium from sulfuric acid leach liquid of zinc displacement residues
The technology of slag sulfuric acid and leaching solution is applied in the field of selective extraction and recovery of gallium germanium indium, which can solve the problems of large damage degree of extractant, high water solubility and high alkali concentration, and achieves good back-extraction effect, good dispersion performance and high recovery rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Aqueous phase material solution: sulfuric acid leaching solution of zinc replacement slag, containing germanium 0.254g / L, gallium 0.311g / L, indium 0.06g / L, iron 1.07g / L, zinc 16.55g / L, nickel 0.58g / L, cobalt 0.87g / L, cadmium 4.6g / L, sulfuric acid concentration of feed liquid 100g / L;
[0040] Extract germanium organic phase: the extractant is 0.2mol / L HBL101+0.1mol / L P507, and the diluent is sulfonated kerosene.
[0041] Extraction and separation: The organic phase and the feed liquid are subjected to single-stage extraction under the condition of a ratio of 1 / 1, the extraction equilibrium time is 10 minutes, and the temperature is 25°C. The experimental results are shown in Table 1.
[0042] Table 1 Extraction rate (%) of metal ions in sulfuric acid leaching solution of zinc replacement slag extracted by HBL101+P507 co-extraction system
[0043]
[0044]
[0045] It can be seen from Table 1 that the HBL101+P507 co-extraction system has excellent extraction selecti...
Embodiment 2
[0047] The aqueous phase material liquid is the extracting germanium raffinate solution in Example 1, containing about 100g / L of sulfuric acid, after diffusion dialysis, the concentration of sulfuric acid in the dialysate is only 5g / L, adding concentrated NaOH solution to adjust the pH value to 3.0 as the extraction of gallium , The raw material solution of indium, the concentration of metal ions remains basically unchanged;
[0048] Gallium indium extraction organic phase: extractant is 0.1mol / L HBL101+0.1mol / L Versatic 10, diluent is No. 260 solvent naphtha.
[0049] Extraction and separation: The organic phase and the feed liquid are subjected to single-stage extraction under the condition of a ratio of 1 / 1, the extraction equilibrium time is 10 minutes, and the temperature is 25°C. The experimental results are shown in Table 2.
[0050] Table 2 HBL101+Ver.10 co-extraction system extraction extraction rate of metal ions in germanium raffinate (%)
[0051]
[0052] It c...
Embodiment 3
[0054] Water phase feed liquid is identical with embodiment 1;
[0055] The organic phase is an extractant of 0.2mol / L HBL101+0.2mol / L P507, and the diluent is aviation kerosene.
[0056] Stripping agent: 0.5mol / L NaOH solution.
[0057] Extraction and separation: the above-mentioned organic phase and the aqueous phase feed liquid are subjected to 3-stage countercurrent extraction under the condition of 1 / 5 of the ratio, and single-stage stripping is performed under the condition of 1 / 1 of the ratio of the stripping agent used for the loaded organic phase. The mixing time for both extraction and back extraction was 10 min, and the temperature was 25 °C. Table 3 shows the experimental results after extraction-stripping reached equilibrium.
[0058] Table 3 HBL101+P507 multistage countercurrent cascade extraction simulation test results
[0059]
[0060] Note: -- not detected
[0061] It can be seen from Table 3 that after three stages of countercurrent extraction, the ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com