Cross-linking type anion membrane and preparation method thereof
An anionic membrane, cross-linked technology, applied in electrical components, circuits, battery electrodes, etc., can solve problems such as the inability to meet the needs of industrialization, and the lack of in-depth reports on the chemical stability of membranes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] In a nitrogen atmosphere, at room temperature, dissolve tetramethylurea in dichloromethane, add oxalyl chloride dropwise, slowly raise the temperature to 60°C for 2 hours, and remove the solvent to obtain a white solid, which is Vilsmeyer salt, (tetramethylurea Urea: dichloromethane: oxalyl chloride = 9.6ml: 20ml: 10.2ml);
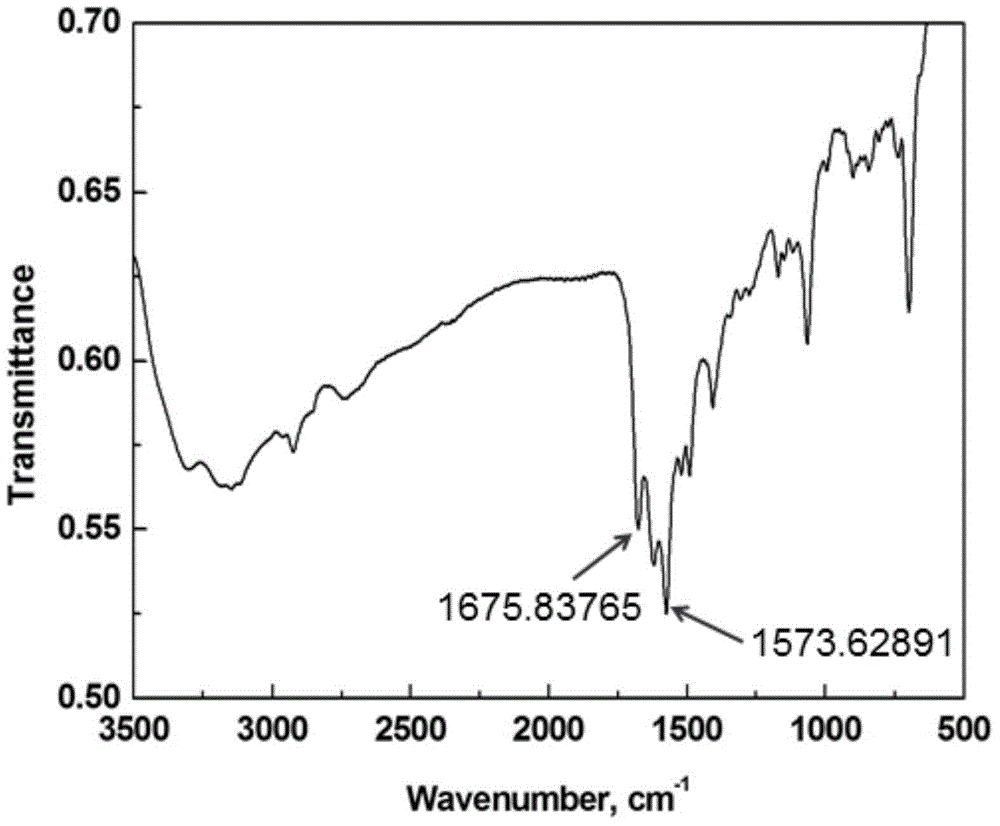
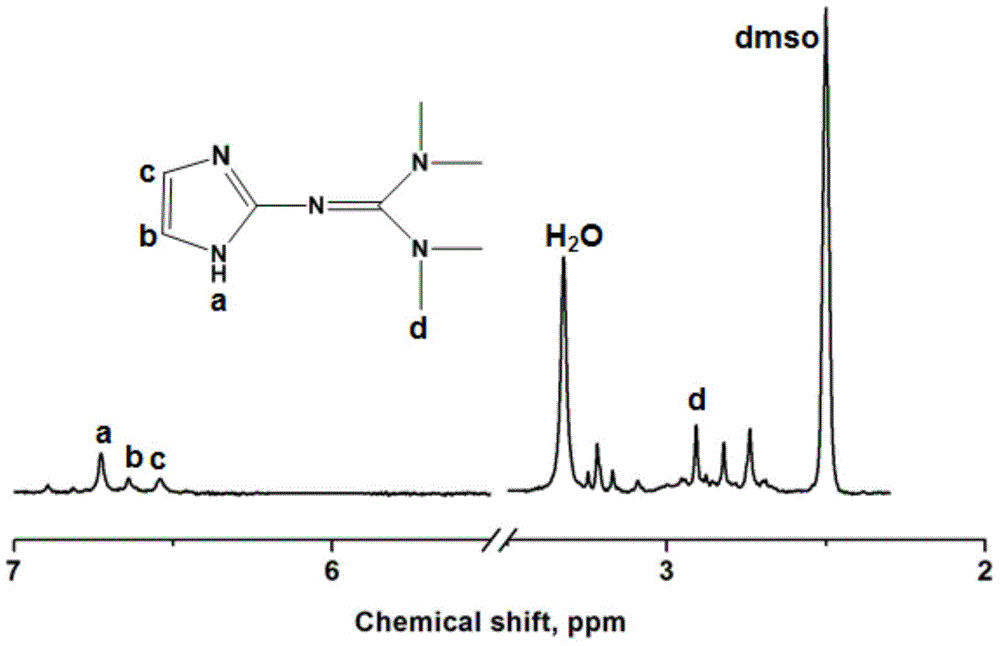
[0045] Dissolve 2 molar parts of Vilsmeyer salt prepared in 10 molar parts of acetonitrile, and slowly add 2 molar parts of 2-aminoimidazole after complete dissolution, and slowly raise the temperature to 90° C. for 24 hours. After the reaction was completed, 2M NaOH aqueous solution was added under ice-bath conditions to adjust the pH value of the reaction mixture to 9-14, and the mixture was vigorously stirred for 4 hours until the system became clear. Separate the organic phase, use 20mL ethyl acetate to extract the inorganic phase 5 times, combine the organic phases, dry with anhydrous sodium sulfate, remove solvent and extractant, obtain 2-imid...
Embodiment 2
[0051] In a nitrogen atmosphere, at room temperature, dissolve tetramethylurea in dichloromethane, add oxalyl chloride dropwise, slowly raise the temperature to 60°C for 2 hours, and remove the solvent to obtain a white solid, which is Vilsmeyer salt, (tetramethylurea Urea: dichloromethane: oxalyl chloride = 9.6ml: 20ml: 10.2ml);
[0052] Dissolve 2 molar parts of Vilsmeyer salt prepared in 10 molar parts of acetonitrile, and slowly add 2 molar parts of 2-aminoimidazole after complete dissolution, and slowly raise the temperature to 90° C. for 24 hours. After the reaction was completed, 2M NaOH aqueous solution was added under ice-bath conditions to adjust the pH value of the reaction mixture to 9-14, and the mixture was vigorously stirred for 4 hours until the system became clear. Separate the organic phase, use 20mL ethyl acetate to extract the inorganic phase 5 times, combine the organic phases, dry with anhydrous sodium sulfate, remove solvent and extractant, obtain 2-imid...
Embodiment 3
[0058] In a nitrogen atmosphere, at room temperature, dissolve tetramethylurea in dichloromethane, add oxalyl chloride dropwise, slowly raise the temperature to 60°C for 2 hours, and remove the solvent to obtain a white solid, which is Vilsmeyer salt, (tetramethylurea Urea: dichloromethane: oxalyl chloride = 9.6ml: 20ml: 10.2ml);
[0059] Get the Vilsmeyer salt of prepared 2 molar parts and be dissolved in the acetonitrile of 10 molar parts, slowly add the 2-aminoimidazole of 2 molar parts after dissolving completely, then add the triethylamine of 2 molar parts (as the acid agent that promotes reaction ), slowly warming up to 90°C for 24 hours. After the reaction was completed, 2M NaOH aqueous solution was added under ice-bath conditions to adjust the pH value of the reaction mixture to 9-14, and the mixture was vigorously stirred for 4 hours until the system became clear. Separate the organic phase, use 20mL ethyl acetate to extract the inorganic phase 5 times, combine the o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Water absorption | aaaaa | aaaaa |
| Water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com