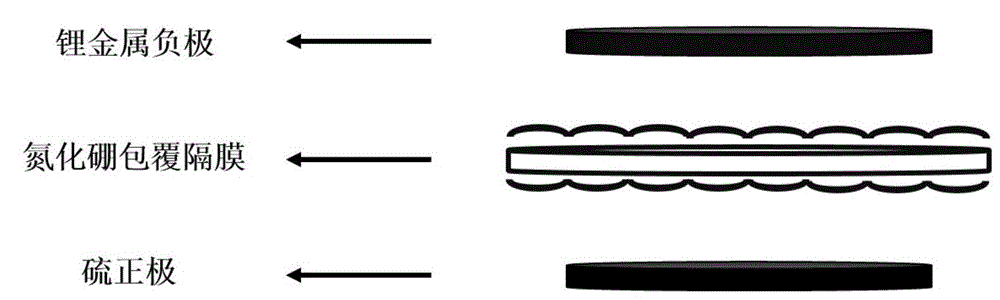
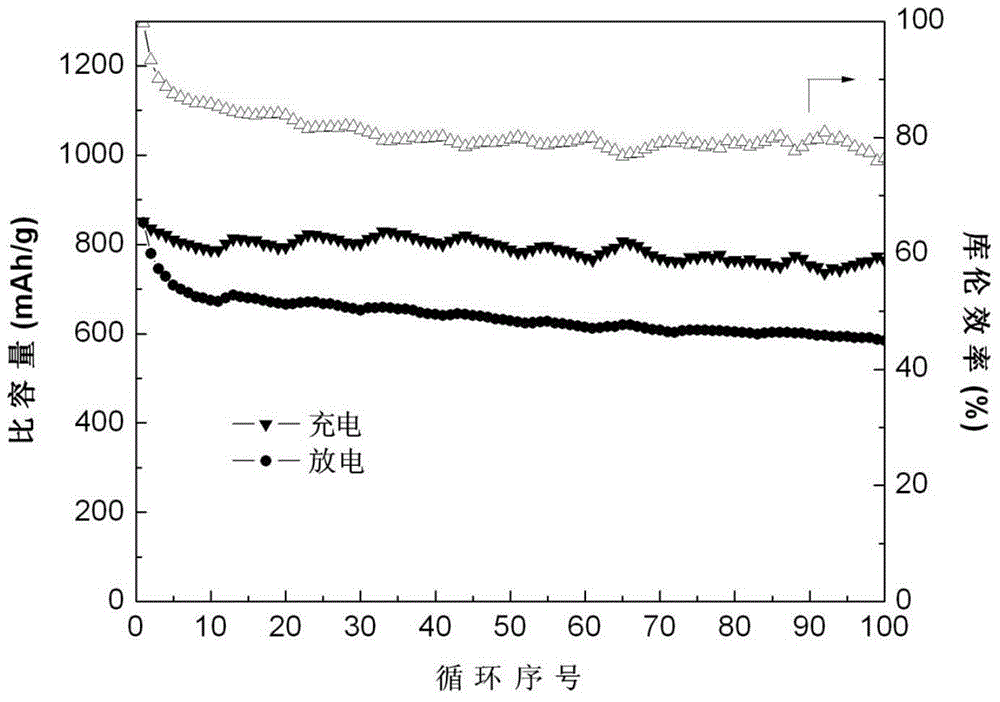
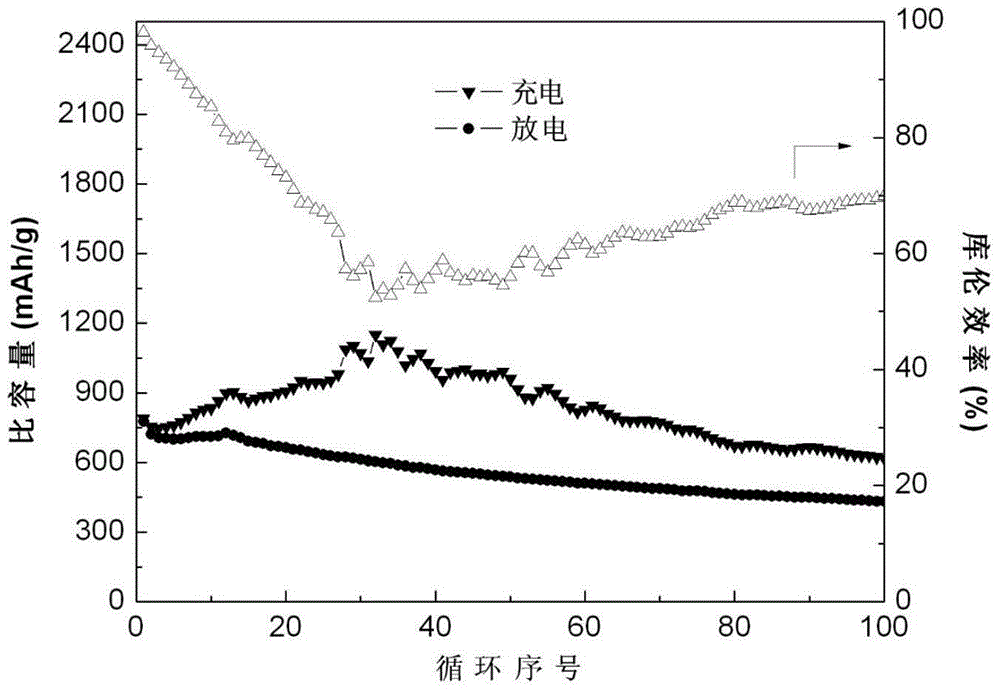
Production method of boron nitride coated diaphragm of lithium-sulfur battery
A technology of boron nitride packs and lithium-sulfur batteries, which is applied in the direction of lithium batteries, battery components, non-aqueous electrolyte batteries, etc., can solve the problem of hindering the discharge reaction between electrolytes and electrode active materials, reducing battery Coulombic efficiency and cycle life, and preparing The method is unfavorable for promotion and universalization, and achieves the effects of suppressing the shuttle effect, ensuring cycle stability, and avoiding overcharging.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Take a piece of Celgard 2325 diaphragm, use MSK-T10 button cell slicer to cut into a disc with a diameter of 19mm, put it in a beaker, use acetone, isopropanol and ethanol to clean it ultrasonically, and then use deionized water Rinse and dry. Take 1 mg of hexagonal boron nitride powder, dissolve it in 50 mL of N-methylpyrrolidone, stir it thoroughly, and sonicate it for 24 hours. After sonication, the solution was transferred to a centrifuge tube and centrifuged at 500 rpm for 10 min. Add 1 mg of polyvinylidene fluoride to the supernatant after centrifugation, and stir thoroughly to obtain boron nitride slurry. Place the washed diaphragm on a flat glass bottom plate, pour the slurry on the diaphragm, and use a four-sided film applicator to manually coat the film with a film thickness of 25um; after drying at 50°C, coat the reverse side of the diaphragm again. After drying again, a boron nitride-coated diaphragm is obtained.
Embodiment 2
[0041] Take a piece of Celgard 2325 diaphragm, use MSK-T10 button cell slicer to cut into a disc with a diameter of 19mm, put it in a beaker, use acetone, isopropanol and ethanol to clean it ultrasonically, and then use deionized water Rinse and dry. Take 5 mg of hexagonal boron nitride powder, dissolve it in 50 mL of N-methylpyrrolidone, stir it thoroughly, and ultrasonicate for 24 hours. After the sonication, the solution was transferred to a centrifuge tube and centrifuged at 1000rpm for 30min. After centrifugation, 3 mg of polyvinylidene fluoride was added to the supernatant, and the boron nitride slurry was obtained after thorough stirring. Take 50mL of the prepared slurry and place it in a beaker, grab a piece of diaphragm with tweezers, soak it in the beaker for 30s, then take it out and let it stand in the air for 60s, put the diaphragm in a drying oven to dry, and then the nitrided Boron coated diaphragm.
Embodiment 3
[0043] Take a piece of Celgard 2325 diaphragm, use MSK-T10 button cell slicer to cut into a disc with a diameter of 19mm, put it in a beaker, use acetone, isopropanol and ethanol to clean it ultrasonically, and then use deionized water Rinse and dry. Take 5 mg of hexagonal boron nitride powder, dissolve it in 100 mL of N-methylpyrrolidone, stir it thoroughly, and ultrasonicate for 24 hours. After the sonication, the solution was transferred to a centrifuge tube and centrifuged at 3000rpm for 30min. Add 1 mg of polyvinylidene fluoride to the supernatant after centrifugation, and stir thoroughly to obtain boron nitride slurry. Take a diaphragm and fix it on the substrate of the spin coater, adjust the speed of the spin coater to 60rpm, slowly inject the boron nitride slurry from the liquid injection port at a uniform speed, then adjust the speed of the spin coater to 300rpm, wait for the spin coating to be uniform, and take the diaphragm out Next, dry. Then spin-coat the reve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com