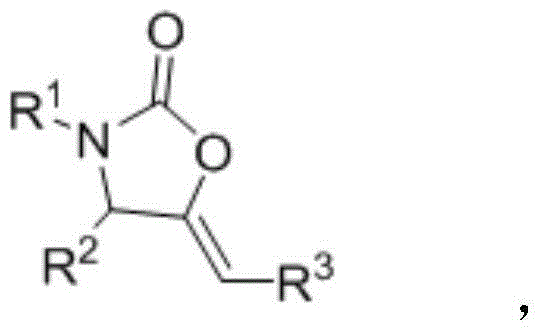
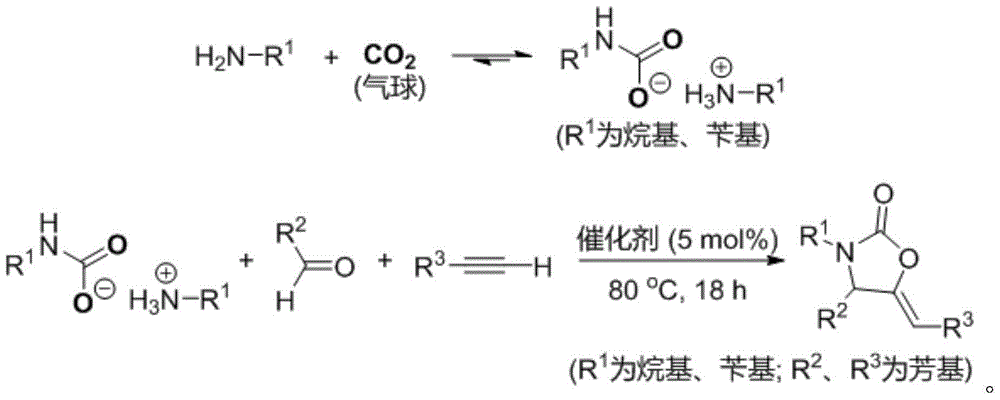
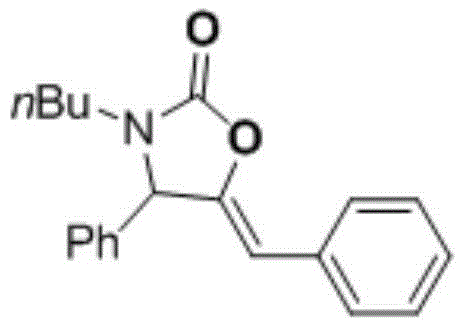
2-oxazolidinone derivative preparation method
A technology of oxazolone and derivatives, which is applied in the field of preparation of 2-oxazolone derivatives, can solve the problems of limited types of 2-oxazolone derivatives, cumbersome synthesis steps, and high price, so as to avoid High-pressure reaction equipment, the effect of improving reaction efficiency and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 20mL of 1-n-butylamine into a 50mL reaction tube, and introduce carbon dioxide gas into it at a flow rate of about 25mL / min. After 5min, a white solid is obtained, which is butylammonium butylcarbamate; in a 10mL reaction tube with magnetic stirring, Add 10 mg of catalyst CuI, 0.4 mL of solvent isopropanol, 2 mmol of phenylacetylene, 2 mmol of benzaldehyde, and 1 mmol of butylammonium butylcarbamate. After sealing the reaction tube, control the reaction temperature at 80°C and react for 18 hours under magnetic stirring. The final product was separated by column chromatography, and the yield of the final product was 84% based on the molar amount of carbon dioxide as 100%.
[0027] The final product is identified by nuclear magnetic resonance, gas chromatography-mass spectrometry, and high-resolution mass spectrometry, and is a 2-oxazolinone derivative according to the present invention. The specific results are as follows:
[0028]
[0029] (Z)-5-benzylidene-3-b...
Embodiment 2
[0032] The terminal alkyne is 4-methylphenylacetylene, other test methods and conditions are the same as in Example 1, and the yield of the final product is 86%.
[0033] The final product is identified by nuclear magnetic resonance, gas chromatography-mass spectrometry, and high-resolution mass spectrometry, and is a 2-oxazolinone derivative according to the present invention. The specific results are as follows:
[0034]
[0035] (Z)-3-butyl-5-(4-methylbenzylidene)-4-phenyloxazolidin-2-one,
[0036] 1 H NMR (CDCl 3 ,400MHz): δ=7.42-7.25(m,7H),7.11-7.09(m,2H),5.37(s,1H),5.21(s,1H),3.54-3.47(m,1H),2.85-2.80 (m,1H),2.31(s,3H),1.47-1.43(m,2H),1.31-1.25(m,2H),0.87(t,3H,J=7.4Hz); 13 C NMR (CDCl 3 ,100.6MHz): δ=155.1,146.9,137.4,136.7,130.6,129.3,129.2,129.1,128.2,127.8,104.4,63.8,41.6,28.9,21.2,19.8,13.6; HRMS(ESI):C 21 h 23 NO 2 Na for [M+Na] + calculated 344.1621, found 344.1666; EI-MS, m / z(%): 194.10(100), 321.20(49)[M + ].
Embodiment 3
[0038] The terminal alkyne is 4-methoxyphenylacetylene, other test methods and conditions are the same as in Example 1, and the yield of the final product is 81%.
[0039] The final product is identified by nuclear magnetic resonance, gas chromatography-mass spectrometry, and high-resolution mass spectrometry, and is a 2-oxazolinone derivative according to the present invention. The specific results are as follows:
[0040]
[0041] (Z)-3-butyl-5-(4-methoxybenzylidene)-4-phenyloxazolidin-2-one,
[0042] 1 H NMR (CDCl 3 ,400MHz): δ=7.59-7.57(m,1H),7.47-7.36(m,3H),7.33-7.31(m,2H),6.84-6.82(m,3H),5.36(s,1H),5.19 (s,1H),3.78(s,1H),3.52-3.46(m,1H),2.85-2.79(m,1H),1.46-1.43(m,2H),1.30-1.26(m,2H),0.87 (t,3H,J=7.8Hz); 13 C NMR (CDCl 3 , 100.6MHz): δ=158.4, 155.1, 145.9, 137.5, 133.0, 129.6, 129.2, 127.7, 126.2, 113.8, 104.0, 63.7, 55.2, 41.5, 28.9, 19.7, 13.6; HRMS(ESI): C 21 h 23 NO 3 Na for [M+Na] + calculated 360.1570, found 360.1587; EI-MS, m / z(%): 210.10(100), 337.20(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com