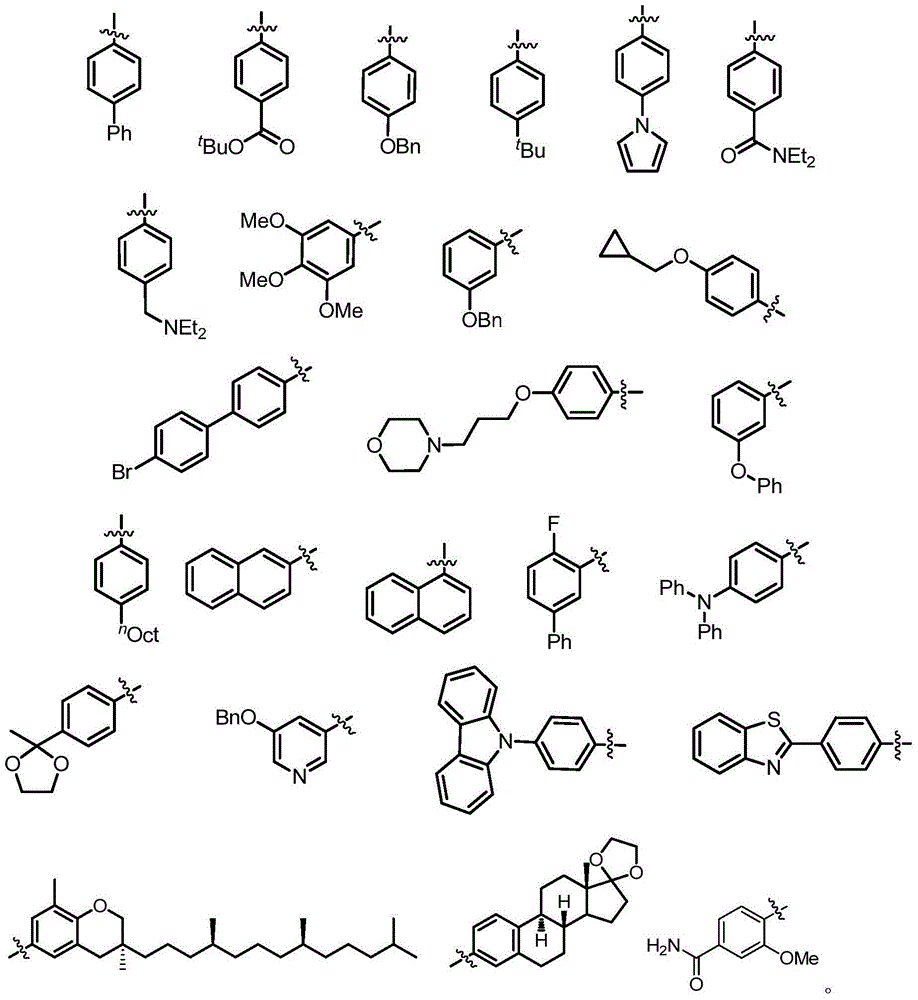
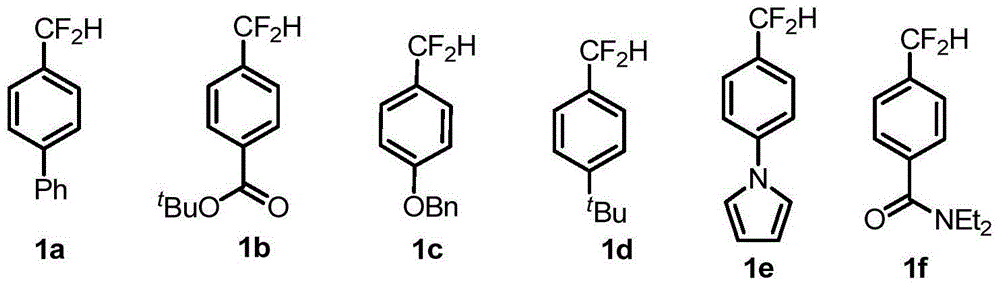
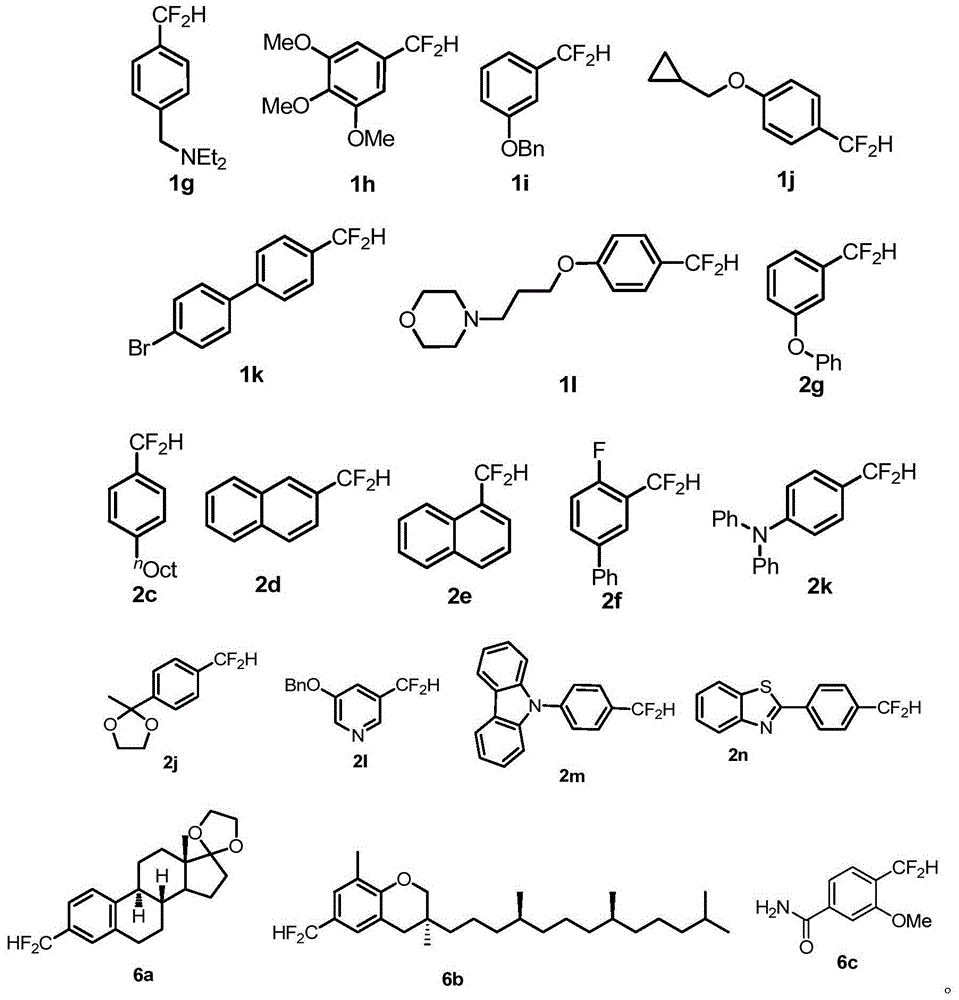
Compound containing difluromethylation and preparation method therefor
A difluoromethyl compound technology, applied in the field of difluoromethyl-containing compounds, can solve problems such as high production costs, unsuitable for industrial production, and long routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The difluoromethylation reaction research of the iodoarene (RI) that palladium participates in embodiment 1
[0072]Under the condition of argon, 4-phenyliodobenzene (0.1mmol), trimethyldifluoromethylsilane, palladium catalyst, additives, ligands and bases were dissolved in a dry deoxygenated solvent in the corresponding proportions in the table , heated to the temperature listed in the table, and after reacting for the time listed in the table, an equal proportion of trifluorotoluene was added as an internal standard, and the yield was determined by fluorine spectrum. See Table 1 for the screening of palladium catalysts, see Table 2 for the screening of additives, see Table 3 for the screening of bases, see Table 4 for the screening of solvents, see Table 5 for the screening of ligands, and see Table 6 for the screening of reaction temperature , see Table 7 for the screening of reaction time, see Table 8 for the screening of palladium catalyst dosage, see Table 9 for t...
Embodiment 2
[0121] The experiment of the difluoromethylation of the iodoarene (RI) of embodiment 2 palladium catalysis
[0122]Under argon atmosphere, iodoarene (0.5mmol), bis(dibenzylideneacetone)palladium (14.5mg, 0.025mmol), 1,1'-bis(diphenylphosphino)ferrocene (28mg, 0.05 mmol), additive SIPrAgCl (53.5 mg, 0.1 mmol), sodium tert-butoxide (96.1 mg, 1.0 mmol) were dissolved in 5 mL of dioxane, and 150 uL of trimethyldifluorosilane was added to the system. After reacting at 80°C for 4 hours, cool to room temperature, add 10 mL of distilled water to quench the reaction, filter through celite and extract the filtrate with dichloromethane (25ml×3), combine the organic phases, dry over anhydrous sodium sulfate, concentrate, and After separation, the corresponding difluoromethylated products 1a-1l were obtained, and the specific yields are shown in Table 12.
[0123] Table 12 Study on the suitability of substrates for difluoromethylated arenes with iodine bands
[0124]
[0125]
[01...
Embodiment 3
[0151] The difluoromethylation reaction research of the brominated arene (RBr) that embodiment 3 palladium participates in
[0152] Under the condition of argon, 4-phenylbromobenzene (0.1mmol), trimethyldifluoromethylsilane, palladium catalyst, additives, ligands and bases were dissolved in the dry deoxygenated solvent in the corresponding proportions in the table , heated to the temperature listed in the table, and after reacting for the time listed in the table, an equal proportion of trifluorotoluene was added as an internal standard, and the yield was determined by fluorine spectrum. See Table 13 for the screening of palladium catalysts, see Table 14 for the screening of additives, see Table 15 for the screening of bases, see Table 16 for the screening of solvents, see Table 17 for the screening of ligands, and see Table 18 for the screening of reaction temperature , see Table 19 for the screening of reaction time, see Table 20 for the screening of palladium catalyst dosag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com