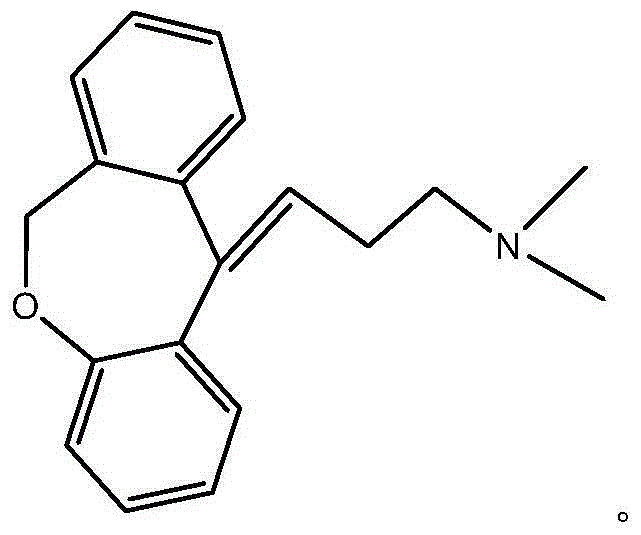
Synthesis method of doxepin hydrochloride
A kind of technology of doxepin hydrochloride and synthesis method, applied in the synthesis field of doxepin hydrochloride, can solve problems such as low yield and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
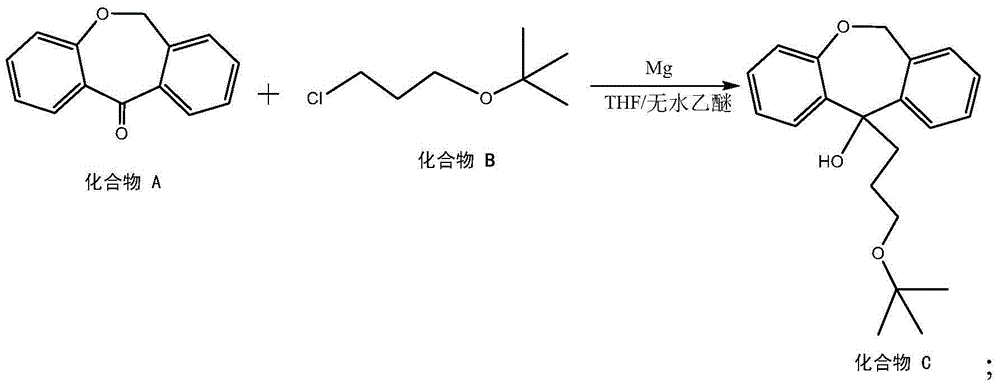
[0031] In a 20L reaction vessel, put 6,11-dihydrodibenzo[b,e]oxepin-11-one, 1.1 times the molar ratio of 6,11-dihydrodibenzo[b,e]oxa 3-chloropropanyl tert-butyl ether of heptin-11-one, magnesium powder twice the mass of 6,11-dihydrodibenzo[b,e]oxepin-11-one, all THF Two-fifths (5 times the mass of THF of 6,11-dihydrodibenzo[b,e]oxepin-11-one), and heated to 35°C to make it react. After the reaction started, the remaining 3 / 5 of THF was added dropwise. After a total reaction time of 5 h, the reaction was stopped. Then the system was cooled and poured into saturated ammonium chloride solution, extracted twice with ethyl acetate, dried with anhydrous sodium sulfate for 5 hours, and the obtained crude product was recrystallized with petroleum ether to obtain hydroxyl compounds.
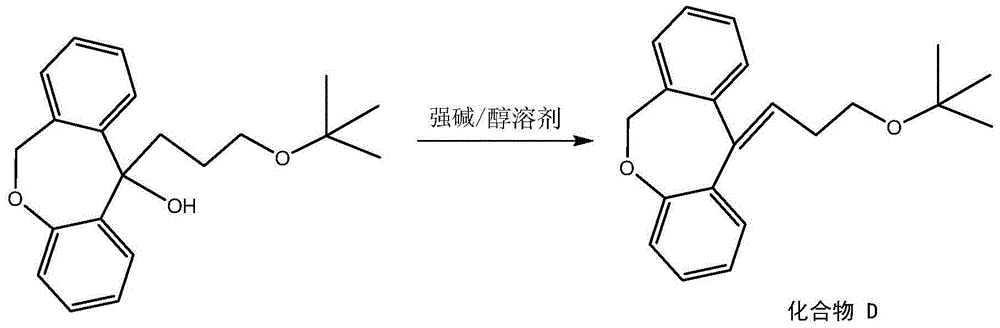
[0032] In a 20L reaction vessel, put the ethanol solution (mass concentration 10wt%) of the above-mentioned hydroxy compound and sodium hydroxide 1.5 times the mass of the hydroxy compound, heat to 65°C...
Embodiment 2
[0037]In a 20L reaction vessel, put 6,11-dihydrodibenzo[b,e]oxepin-11-one, 1.5 times the molar ratio of 6,11-dihydrodibenzo[b,e]oxa 3-chloropropanyl tert-butyl ether of heptin-11-one, magnesium powder with 2.4 times the mass of 6,11-dihydrodibenzo[b,e]oxepin-11-one, all THF Two-fifths (5 to 7 times the mass of THF of 6,11-dihydrodibenzo[b,e]oxepin-11-one), and heated to 40°C to make it react. After the reaction started, the remaining 3 / 5 of THF was added dropwise. After a total reaction time of 2 h, the reaction was stopped. Then the system was cooled and poured into saturated ammonium chloride solution, extracted twice with ethyl acetate, dried with anhydrous sodium sulfate for 5 hours, and the obtained crude product was recrystallized with petroleum ether to obtain hydroxyl compounds.
[0038] Put the ethanol solution (mass concentration 70wt%) of the above-mentioned hydroxy compound and sodium hydroxide 5 times the mass of the hydroxy compound in a 20L reaction vessel, he...
Embodiment 3
[0043] In a 20L reaction vessel, put 6,11-dihydrodibenzo[b,e]oxepin-11-one, 1.3 times the molar ratio of 6,11-dihydrodibenzo[b,e]oxa 3-chloropropanyl tert-butyl ether of heptin-11-one, magnesium powder with 2.2 times the mass of 6,11-dihydrodibenzo[b,e]oxepin-11-one, all THF Two-fifths (5-7 times the mass of THF of 6,11-dihydrodibenzo[b,e]oxepin-11-one), and heated to 38°C to make it react. After the reaction started, the remaining 3 / 5 of THF was added dropwise. After a total reaction time of 3.5 h, the reaction was stopped. Then the system was cooled and poured into saturated ammonium chloride solution, extracted twice with ethyl acetate, dried with anhydrous sodium sulfate for 5 hours, and the obtained crude product was recrystallized with petroleum ether to obtain hydroxyl compounds.
[0044] Put the ethanol solution (mass concentration 40wt%) of the above-mentioned hydroxy compound and sodium hydroxide 3 times the mass of the hydroxy compound in a 20L reaction vessel, he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com