Cefaclor compound, medicine composition of cefaclor compound and bromhexine hydrochloride, and preparation of cefaclor compound
A technology of bromhexine hydrochloride and cefaclor, applied in the field of medicine, can solve problems such as research on bioavailability of preparations, and achieve the effects of good clinical treatment effect, good stability and improved synergistic drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 cefaclor trihydrate
[0040] 1. Dissolve the cefaclor crude product in water with a pH of 3.0 and a temperature of 40°C;
[0041] 2. While stirring, add a mixed solvent of diethyl ether and n-hexane with a volume ratio of 2:1; the stirring speed is 1200 rpm; the weight of the mixed solvent is 4 times the weight of the cefaclor aqueous solution, and the adding speed is 30 ml / min;
[0042] 3. After adding the mixed solvent, cool down to -1°C with a cooling rate of 4°C / hour; adjust the pH value to 4.5, obtain crystals and then stand for crystallization; filter, wash, and vacuum dry for 2 hours to obtain cefaclor tris Hydrate.
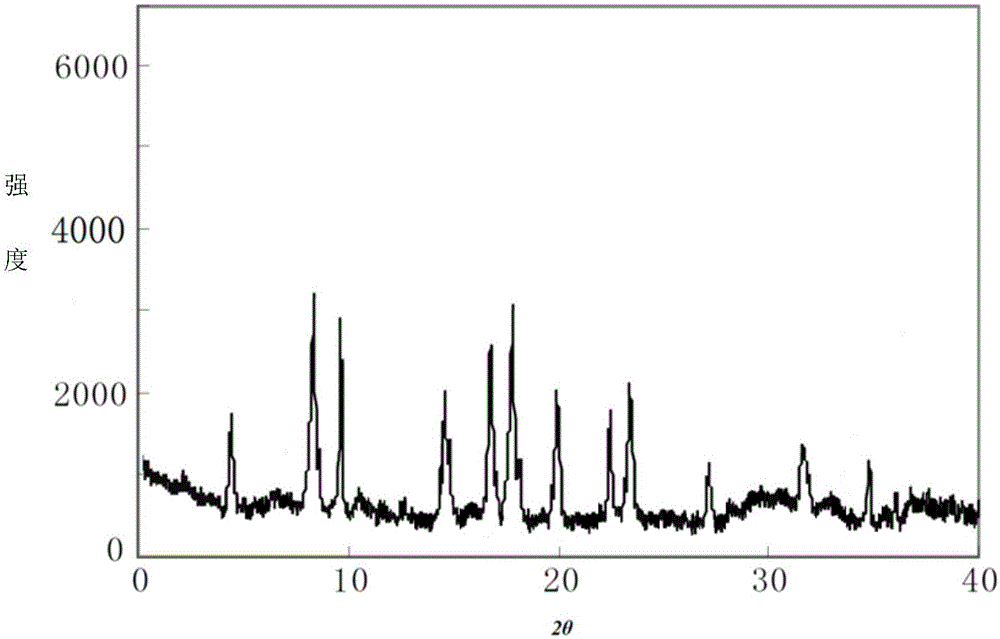
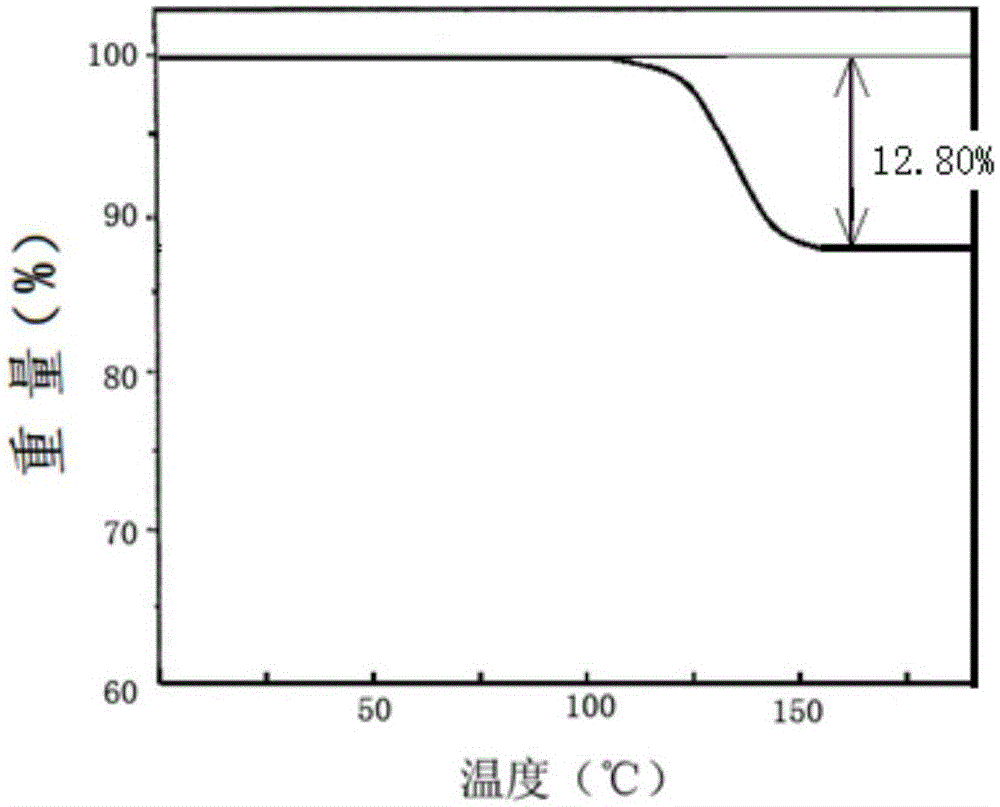
[0043] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.96%, and the yield is 94.0%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 1 As shown, the thermogravimetric analysis diagram is shown as figure 2 Shown; Elem...
Embodiment 2
[0045] The preparation of embodiment 2 cefaclor trihydrate
[0046] 1. The cefaclor crude product is dissolved in water with a pH of 2.5 and a temperature of 35°C;
[0047] 2. While stirring, add a mixed solvent of diethyl ether and n-hexane with a volume ratio of 2:0.8; the stirring speed is 1000 rpm; the weight of the mixed solvent is 3 times the weight of the cefaclor aqueous solution, and the adding speed is 20 ml / min;
[0048] 3. After adding the mixed solvent, cool down to 0°C with a cooling rate of 5°C / hour; adjust the pH value to 5.0, obtain crystals and then stand for crystallization; filter, wash, and vacuum dry for 4 hours to obtain cefaclor trihydrate thing.
[0049]The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.95%, and the yield is 93.5%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 1 As shown, the thermogravimetric analysis diagram is shown as figure 2 Sho...
Embodiment 3
[0050] The preparation of embodiment 3 cefaclor trihydrate
[0051] 1. The cefaclor crude product is dissolved in water with a pH of 3.0 and a temperature of 38°C;
[0052] 2. While stirring, add a mixed solvent of diethyl ether and n-hexane with a volume ratio of 4:1; the stirring speed is 900 rpm; the weight of the mixed solvent is 3 times the weight of the cefaclor aqueous solution, and the adding speed is 20 ml / min;
[0053] 3. After adding the mixed solvent, lower the temperature to -1°C, and the cooling rate is 4°C / hour; adjust the pH value to 4.5, obtain crystals and then stand for crystallization; filter, wash, and vacuum dry for 3 hours to obtain cefaclor tris Hydrate.
[0054] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.96%, and the yield is 94.2%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows: figure 1 As shown, the thermogravimetric analysis diagram is shown as fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com