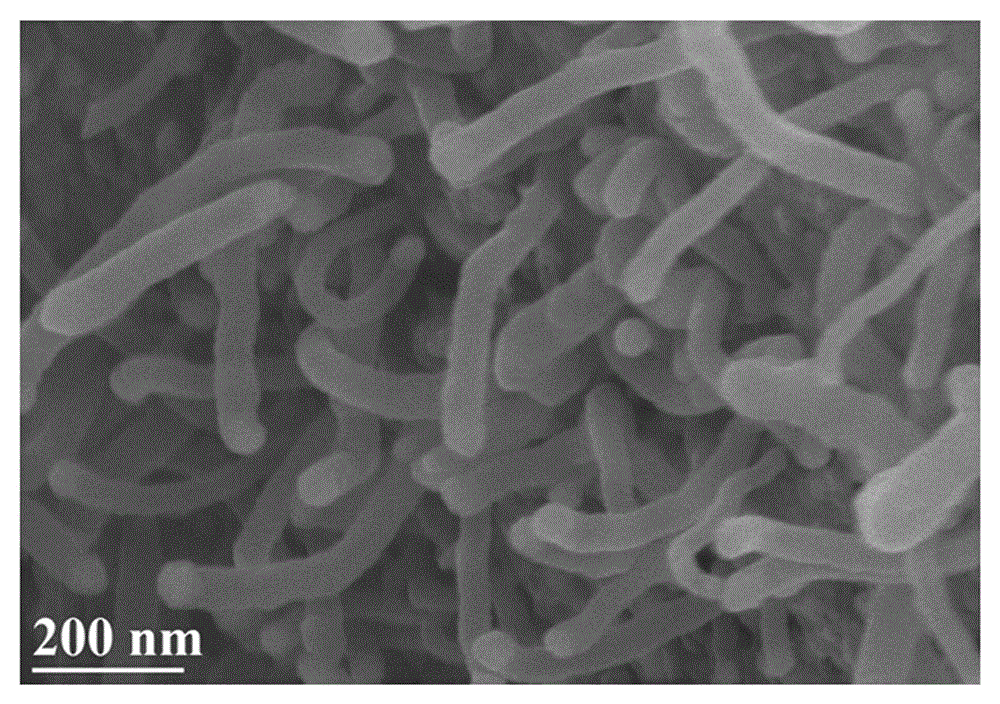
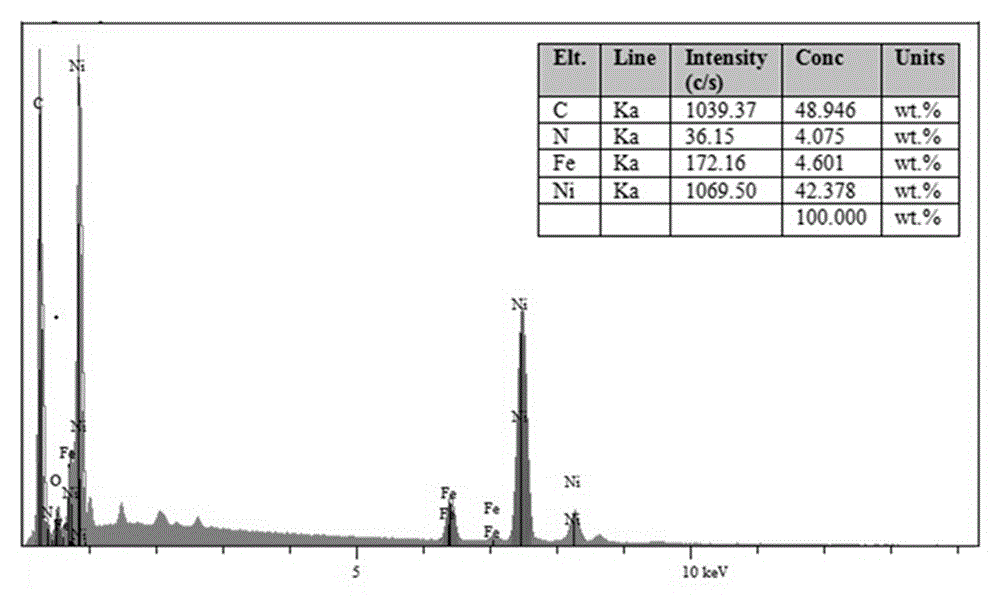
Nitrogen-doped carbon nanotube nickel-iron coated oxygen evolution catalytic material for water electrolysis and application
A nitrogen-doped carbon, catalytic material technology, applied in the electrolysis process, electrolysis components, physical/chemical process catalysts, etc., can solve the problems of strict equipment requirements, complex synthesis process, dangerous operation, etc. The effect of large surface area and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A nitrogen-doped carbon nanotube-coated nickel-iron electrolytic oxygen evolution catalytic material, whose expression is Ni 0.9 Fe 0.1 CN 0.07 , using the in-situ solid-phase method for one-step synthesis, the specific steps are: take 1.591g nickel acetate, 0.27g ferric chloride hexahydrate, 9.61g citric acid and 5.328g thiourea (the molar ratio is 9:1:50:70) Grind and mix well in a mortar, put into a porcelain boat, and use N at a flow rate of 100mL / min 2 Under protection, it is calcined at 700°C for 5 hours, and cooled naturally to obtain the desired product, which is then used as an anode oxygen evolution catalytic material for electrolysis of water.
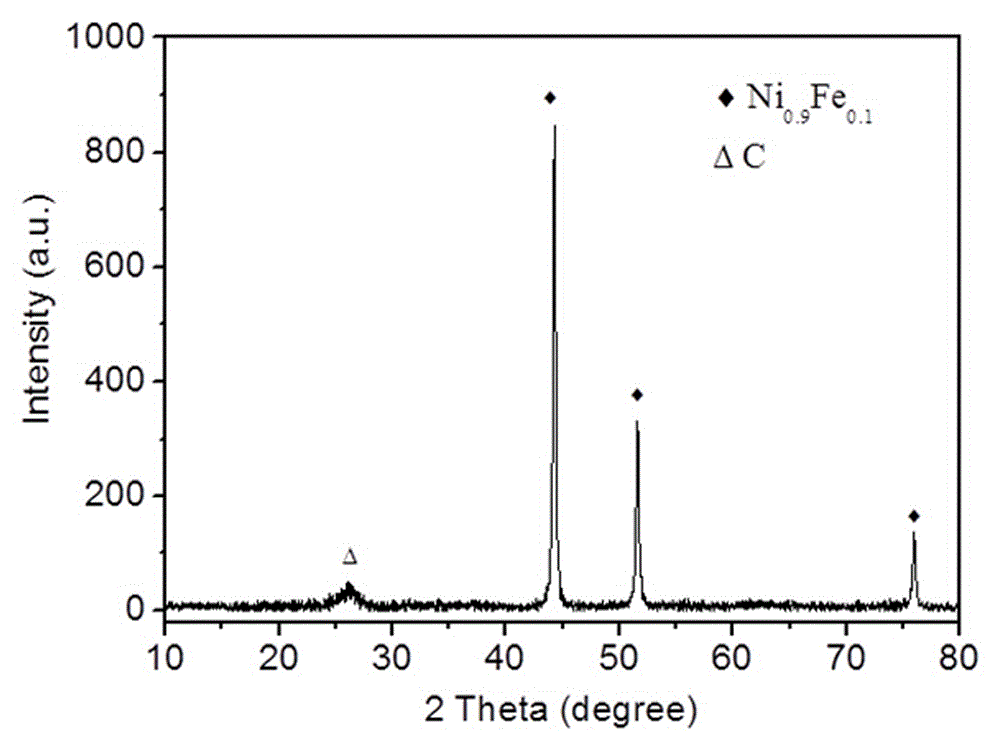
[0024] figure 1 is the Ni 0.9 Fe 0.1 CN 0.07 X-ray diffraction pattern of the material. The analysis results show that the diffraction peaks corresponding to 44.3°, 51.5° and 76.1° in the spectrum belong to Ni 0.9 Fe 0.1 The diffraction peak of , and the diffraction peak corresponding to 26.5° is attributed t...
Embodiment 2
[0032] A nitrogen-doped carbon nanotube-coated nickel-iron electrolytic oxygen evolution catalytic material, whose expression is Ni 0.9 Fe 0.1 CN 0.1 , using the in-situ solid-phase method for one-step synthesis, the specific steps are: take 1.591g nickel acetate, 0.27g ferric chloride hexahydrate, 9.61g citric acid and 7.612g thiourea (the molar ratio is 9:1:50:100) Grind and mix evenly in a mortar, put into a porcelain boat, and use N at a flow rate of 50mL / min 2 Under protection, calcining at 900° C. for 1 h, cooling naturally to obtain the desired product, and then using the obtained product as an anode oxygen evolution catalytic material for electrolyzing water.
[0033] The test results show that: the prepared Ni 0.9 Fe 0.1 CN 0.1 The specific surface area of the nanocomposite is 316.8m2 / g, and its oxygen evolution reaction onset potential is 1.456VvsRHE, at 10mA / cm 2 The oxygen evolution overpotential under the current density is 269mV, the Tafel slope of the ox...
Embodiment 3
[0035] A nitrogen-doped carbon nanotube-coated nickel-iron electrolytic oxygen evolution catalytic material, whose expression is Ni 0.9 Fe 0.1 CN 0.05 , using in-situ solid-phase method for one-step synthesis, the specific steps are: take 1.591g of nickel acetate, 0.27g of ferric chloride hexahydrate, 9.61g of citric acid and 3.806g of thiourea (the molar ratio is 9:1:50:50) Grind and mix evenly in a mortar, put into a porcelain boat, and use N at a flow rate of 10mL / min 2 Under protection, calcining at 600° C. for 10 h, cooling naturally to obtain the desired product, and then using the obtained product as an anode oxygen evolution catalytic material for electrolyzing water.
[0036] The test results show that: the prepared Ni 0.9 Fe 0.1 CN 0.05 The specific surface area of the nanocomposite is 296.6m 2 / g, the onset potential of the oxygen evolution reaction is 1.467VvsRHE, at 10mA / cm 2 The oxygen evolution overpotential under the current density is 283mV, the Tafel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com