Rapid detection of Listeria monocytogenes through combined nanometer immunomagnetic bead technology-colloidal gold chromatography
A technology of Listeria monocytogenes and immune magnetic beads, which is applied in measuring devices, analytical materials, instruments, etc., can solve the problems of complex operation process, low sensitivity, time-consuming and labor-intensive biological detection methods, and achieve high sensitivity and less time-consuming , The effect of low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
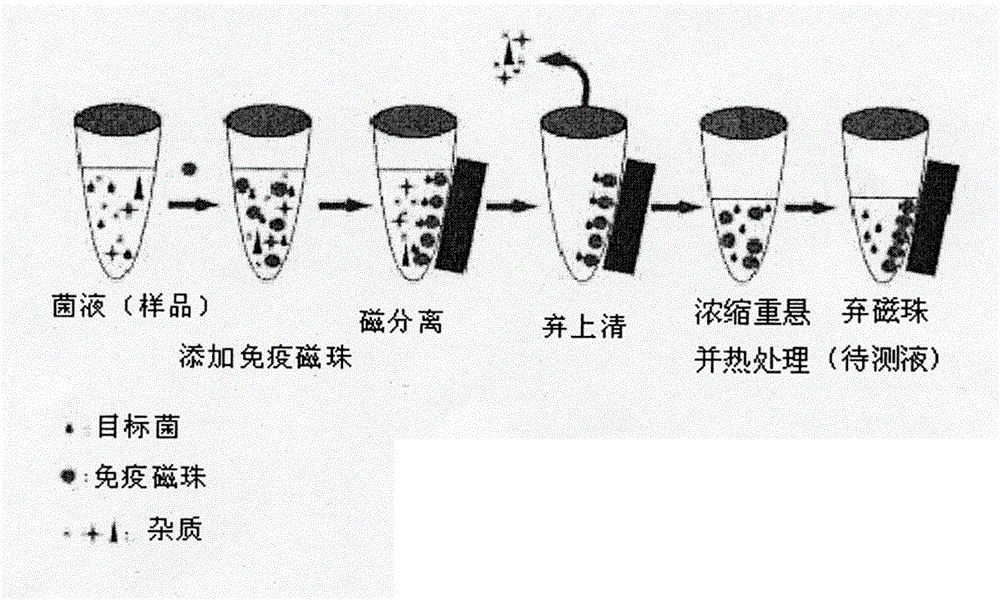
[0029] Implementation Case 1 Preparation of Immunomagnetic Beads and Capture of Listeria Monocytogenes Detection
[0030] 1. Preparation of nano-immunomagnetic beads
[0031] (1) Antibody preparation and purification: 8-week-old healthy BALB / c female mice were immunized with autoclaved Listeria monocytogenes as an antigen, and monoclonal antibodies were prepared and screened by conventional hybridoma technology and limiting dilution method Cell lines, and then the specific antibody cell lines against Listeria monocytogenes were propagated and cultured, and injected into mice to prepare ascites, and the obtained ascites was purified by octanoic acid-ammonium sulfate precipitation; Listeria monocytogenes was treated with high pressure sterilization The bacterium was used as an antigen to immunize New Zealand white rabbits, and the obtained anti-Listeria monocytogenes serum was purified by ammonium sulfate precipitation, and the obtained polyclonal antibody was stored at -80 de...
Embodiment example 2
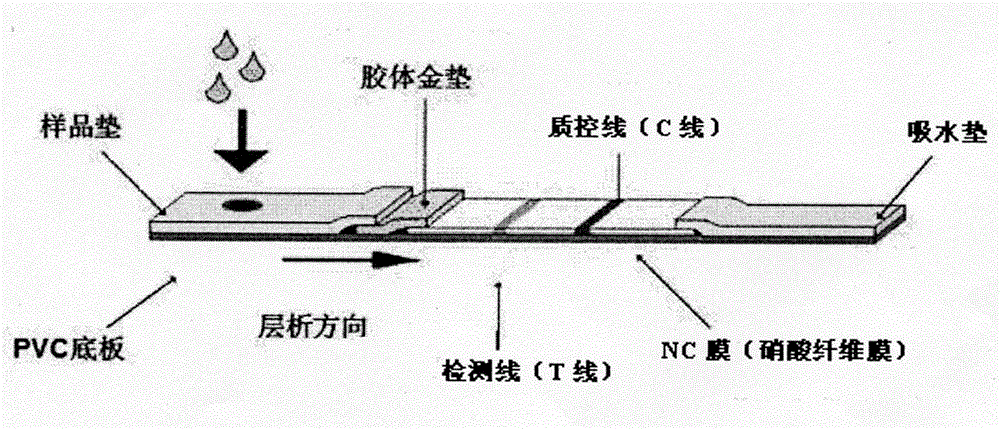
[0041] Implementation Case 2 Preparation of Colloidal Gold Immunochromatography Test Strip and Sample Detection
[0042] 1. Preparation of colloidal gold test strips
[0043] (1) Preparation of colloidal gold: Take 100mL of 0.01% HAuCl4 aqueous solution in a beaker, add 2mL of 1% trisodium citrate when heating and boiling, continue heating and boiling for 5min, add ultrapure water to 100mL after cooling, and prepare 40nm colloidal gold solution;
[0044] (2) Treatment of the conjugation pad: Label another anti-Listeria monoclonal antibody 3B10 prepared by pairing with the magnetic bead-conjugated monoclonal antibody 1B12 on the colloidal gold particles, so that the marking liquid is sprayed on the Combined with pads for later use;
[0045] (3) Coating of chromatographic membrane: use an automatic spraying machine to spray specific concentrations of polyantiserum and goat anti-mouse IgG on the T line and C line of the chromatographic membrane at a speed of 0.9 μL / cm When s...
Embodiment example 3
[0049] Implementation Case 3 Detection of Cultured Samples
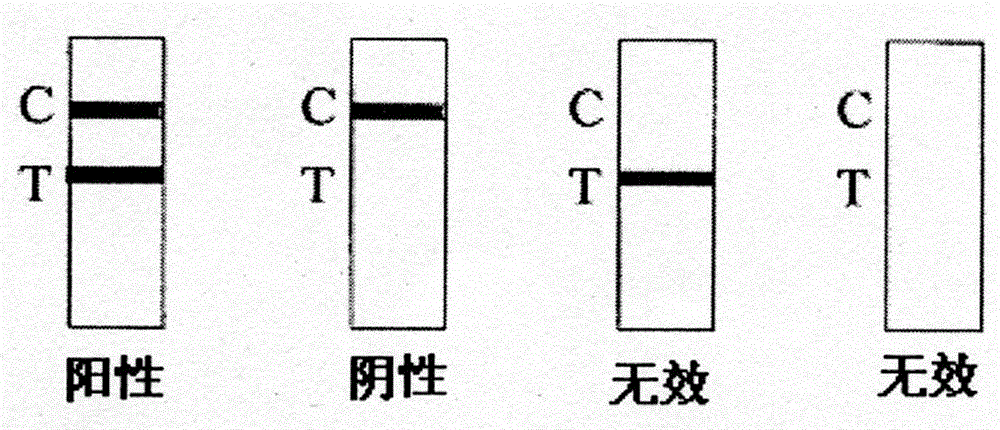
[0050] Dilute the LM bacteria solution cultured for 12 hours to 0, 10, 50, 10 2 ,10 3 ,10 4 ,10 5 CFU / ml, 1 mL each was added to a centrifuge tube, and 0.1 mg of self-made immunomagnetic beads were added to each tube. At room temperature, incubate on a rotary mixer for 30 minutes, resuspend in 1 mL of LPB after magnetic separation, bathe in 85°C water for 15 minutes, suck out the supernatant after magnetic separation, and add 100 μL of the supernatant and the control bacteria solution to the test strips for sampling In the hole, observe the color development of the detection line and quality control line of the test strip for 5-15 minutes.
[0051] Such as Figure 4 As shown, after immunomagnetic bead separation and enrichment reaction, the concentration was 10 3 CFU / mL and 10 2 The test result of the CFU / mL bacterial solution was strongly positive, and the test result was weakly positive at a concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com