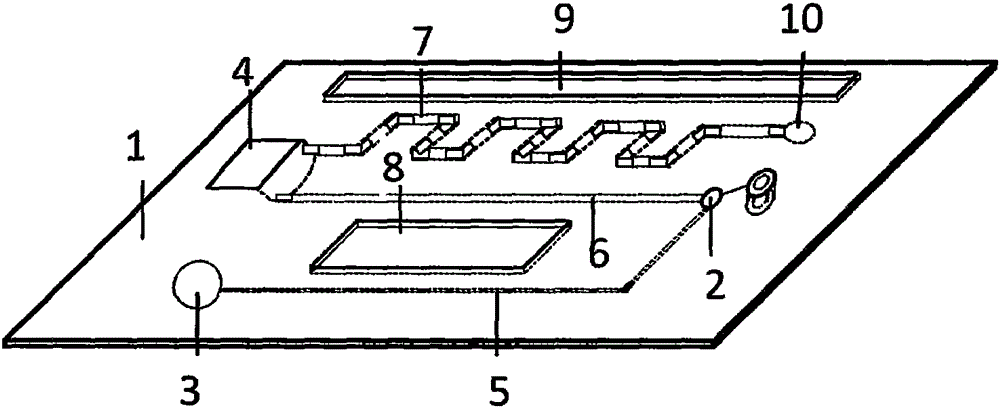
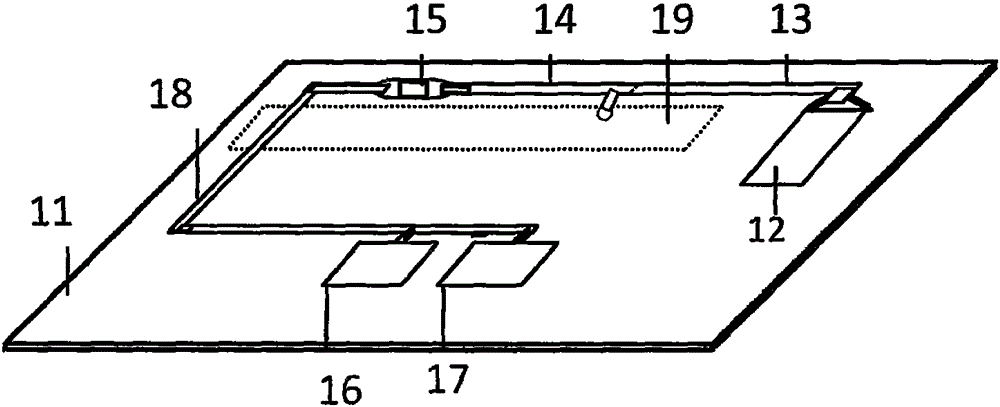
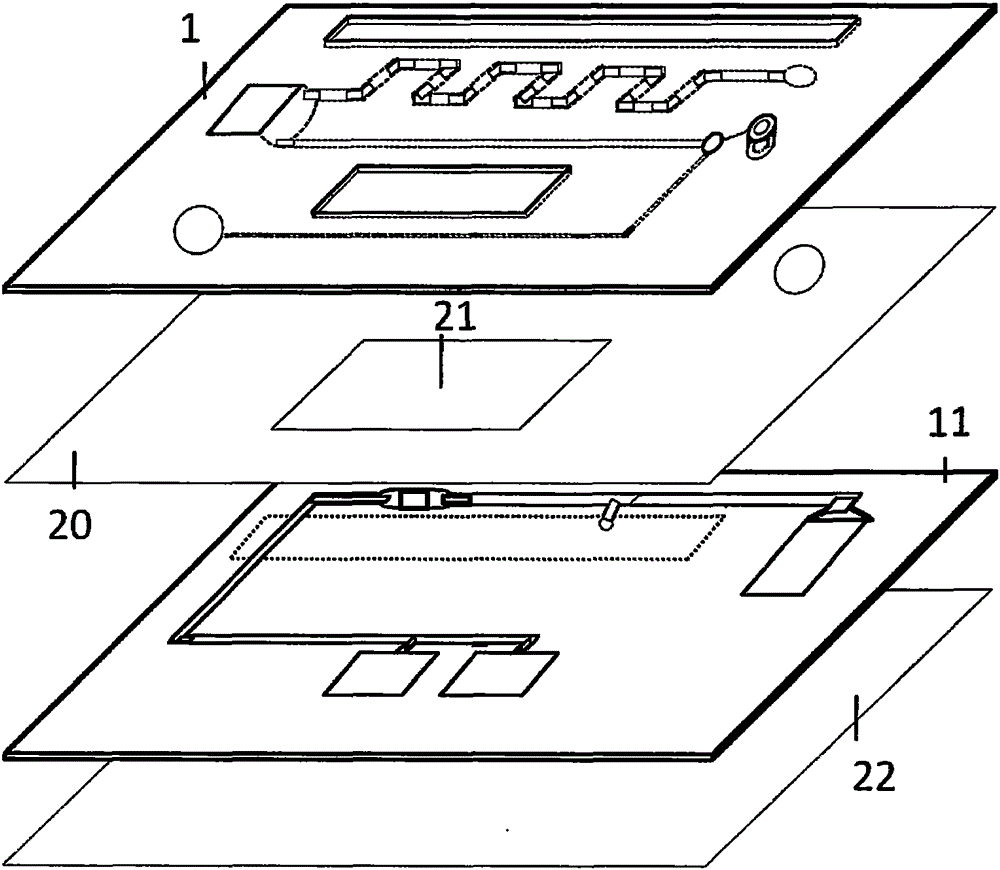
Magnetic particulate chemiluminescent micro-fluidic chip for quantitatively detecting myohemoglobin
A microfluidic chip and myoglobin technology, which is applied in the field of clinical medical testing, can solve problems such as the difficulty in realizing micro-sample detection, insufficient mixing of the reaction system, and the complexity of the microfluidic system, so as to reduce non-specific interference and cost Low, the effect of improving the efficiency of immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: horseradish peroxidase-luminol (HRP-luminol) system is used for the detection of myoglobin
[0055] 1. Fabrication of microfluidic chip
[0056] 1) Antibody labeling: i) Enzyme-labeled antibody: Weigh 5 mg of HRP and dissolve in 1 ml of distilled water; add 0.2 ml of freshly prepared 0.1M NaIO4 solution to the supernatant, and stir for 20 minutes at room temperature in the dark; put the above solution into a dialysis bag , dialyze against 1mM pH4.4 sodium acetate buffer, overnight at 4°C; add 20μl of 0.2M pH9.5 carbonate buffer to raise the pH of the above hydroformylated HRP to 9.0-9.5, then immediately add 10mg of myoglobin antibody , in 1ml of 0.01M carbonate buffer, stir gently at room temperature for 2 hours in the dark; add 0.1ml of newly prepared 4mg / ml NaBH4 solution, mix well, and then place at 4°C for 2 hours; put the above solution into a dialysis bag , dialyze against 0.15MpH7.4PBS, overnight at 4°C; add an equal volume of saturated ammonium s...
Embodiment 2
[0064] Embodiment 2: alkaline phosphatase-adamantane (ALP-AMPPD) system is used for the detection of myoglobin
[0065] 1. Fabrication of microfluidic chip
[0066]1) Antibody labeling: i) Enzyme-labeled antibody: 2.5mgALP (50IU / mg), add 200uL of 100mM PB (pH6.8) containing 1.25% glutaraldehyde, mix well, and react overnight at room temperature; Stir, dialyze to 50mMPBS (pH7.2), 12 hours, change the medium 4 times; dissolve 1.5mg myoglobin antibody in 100uL 1M carbonate solution (pH9.0); add the activated AP into the prepared protein liquid , mix well, react at 4°C for 24 hours, add 10 μL of 200mM lysine solution, mix well, react at 22°C for 2 hours; times; centrifuge, take the supernatant, with 50mMTB7.4+0.6%BSA+0.05%NaN 3 Dilute to the desired concentration and store at -20°C. ii) Magnetically labeled antibody: accurately pipette 30 μl of streptavidin-labeled magnetic beads with a concentration of 1 mg / ml, wherein the functionalized magnetic particles are Fe 3 o 4 As th...
Embodiment 3
[0074] Example 3: Magnetic Particle Size Screening
[0075] The particle size of magnetic microspheres is small, the specific surface area is large, and the surface contains active groups, so the coupling capacity is large, but the size of magnetic particles is too small to be conducive to magnet collection, so the magnetic particle size screening is carried out.
[0076] Refer to Example 2 for other experimental conditions, and the particle size of the magnetic particles is determined according to the following scheme.
[0077] Magnetic particle sizes of 0.1 μm, 0.5 μm, 1.8 μm, 2 μm, 3 μm, and 10 μm were selected to label the anti-C-reactive protein antibody. The permanent magnet whose magnetic size has been optimized is used in the detection to fix the height of the magnet.
[0078] The experimental results are as follows:
[0079] The particle size of magnetic particles increases sequentially from 0.1μm, 0.5μm, 1.8μm, 2μm, and 3μm. The interference increases at 3μm and de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com