Bionic double-chain phospholipid film monolithic column, and making method and application thereof
A double-chain phospholipid, monolithic column technology, applied in the field of chromatographic separation, can solve the problems of inability to effectively simulate the phospholipid environment of cell membranes, unfavorable orderly arrangement of PC functional groups, and decreased ability to predict the effect of drug membranes, etc., so as to overcome residual amino groups and silanol groups. , good permeability, good effect of acid and alkali resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
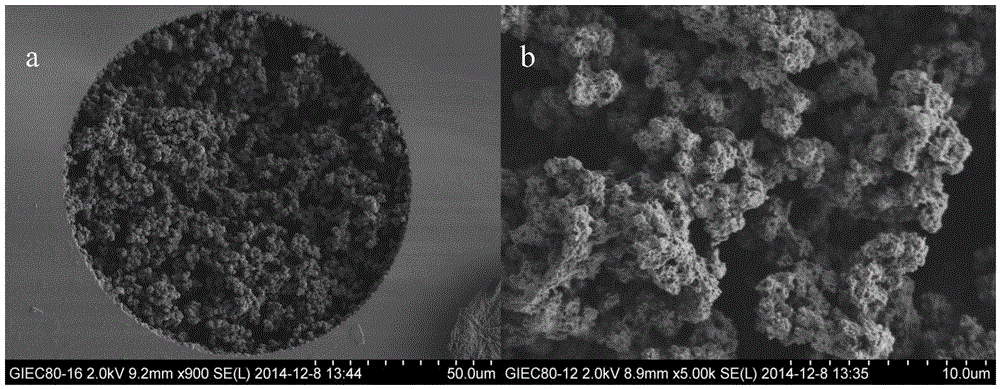
Image
Examples
Embodiment 1
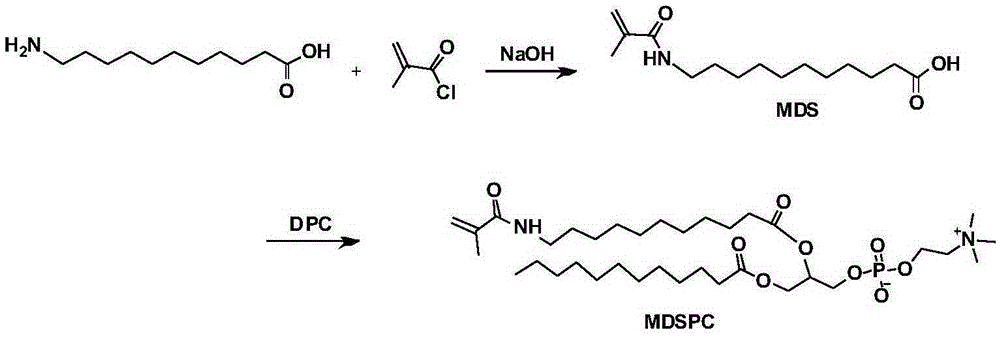
[0036] Preparation of the functional monomer 1-dodecanoyl-2-(11-methacrylamide-N-undecanoyl)-SN-glycerol-3-phosphocholine (MDSPC) in this example:
[0037] (1) In a dry three-necked flask, add 4g of 11-aminoundecanoic acid, 50mL of ethanol, 7mL of water, add 3g of sodium hydroxide at room temperature, stir until the mixture becomes clear, the reaction temperature is lowered to 0°C, slowly drop 2.7mL of methacryloyl chloride solution, continue to react for 3h, then return to room temperature and stir overnight. After the reaction stopped, the reaction liquid was concentrated under reduced pressure and the pH was adjusted to 2.0 with 1 mol / L dilute hydrochloric acid, and a large amount of white flocs were found to be formed. Finally, the floc was vacuum filtered and dried to obtain 3 g of intermediate 11-methacrylamide-N-undecanoic acid (MDS). NMR identified as: 1 HNMR (300MHz, CDCl 3 )δ1.29(m,12H),1.49-1.65(m,4H),1.96(s,3H),2.32-2.37(t,2H),3.27-3.34(m,2H),5.31-5.32(t, 1H), ...
Embodiment 2
[0042] Preparation of the biomimetic double-stranded phospholipid membrane monolithic column in this example:
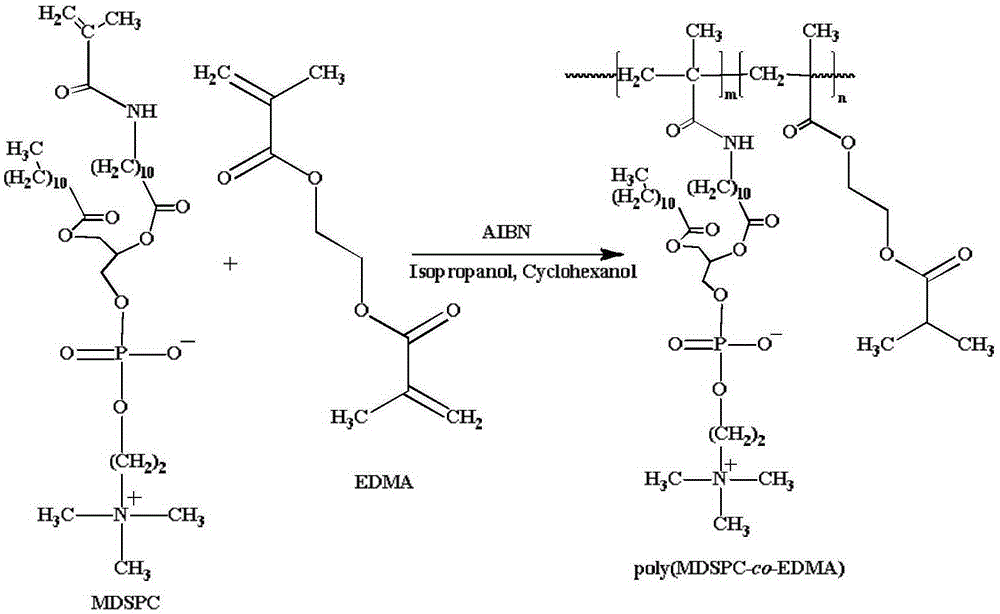
[0043] The 1-dodecanoyl-2-(11-methacrylamide-N-undecanoyl)-SN-glycero-3-phosphocholine (MDSPC) synthesized in Example 1, and the cross-linking agent ethylene glycol Alcohol dimethacrylate (EDMA), porogen isopropanol (Isopropanol) and cyclohexanol (Cyclohexanol) mixture and initiator azobisisobutyronitrile (AIBN) were mixed, ultrasonic degassed for 5 minutes, poured into a modified quartz capillary column at a reaction temperature of 60°C for 12 hours, then connected to a high-pressure pump and rinsed with methanol to obtain a biomimetic double-stranded phospholipid membrane monolithic column (poly(MDSPC-co-EDMA)). The schematic diagram of the above reaction is shown in figure 2 shown. Wherein the mass ratio of MDSPC and crosslinking agent is 45:55, the mass ratio of isopropanol and cyclohexanol in porogen is 83.3:16.7, the sum of MDSPC and crosslinking agent quali...
Embodiment 3
[0047] Preparation of the biomimetic double-stranded phospholipid membrane monolithic column in this example:
[0048] The 1-dodecanoyl-2-(11-methacrylamide-N-undecanoyl)-SN-glycero-3-phosphocholine (MDSPC) synthesized in Example 1, and cross-linking agent (B Glycol dimethacrylate, EDMA), porogen (isopropanol mixed with cyclohexanol) and initiator (azobisisobutyronitrile, AIBN) were mixed, ultrasonic degassed for 5 minutes, poured into modified In the quartz capillary column, the reaction temperature is 60°C, react for 12 hours, connect the high-pressure pump and wash with acetonitrile, and obtain the biomimetic double-stranded phospholipid membrane monolithic column. Wherein the mass ratio of MDSPC and crosslinking agent is 45:55, the mass ratio of isopropanol and cyclohexanol in porogen is 83.3:16.7, the sum of MDSPC and crosslinking agent quality, and the mass ratio of porogen is 34.43:65.57, the mass of the initiator is 1% of the sum of the mass of MDSPC and the crosslink...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com