Esomeprazole-magnesium-containing enteric pellet capsule
A technology of enteric-coated pellets and capsules of esomeprazole magnesium, which is applied in the field of pharmaceutical preparations and can solve the problems of high content of degradation impurities, poor quality stability, and low content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
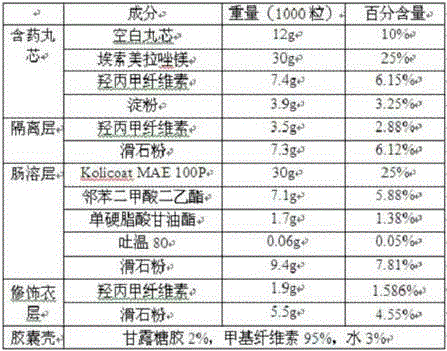
[0175]
[0176] In this example, the blank pellet core is a sucrose-type pellet core purchased from Hangzhou Gaocheng Bio-Nutrition Technology Co., Ltd.
[0177] Preparation method: Dry mix esomeprazole magnesium, part of hypromellose, lactose, sodium lauryl sulfate and disodium hydrogen phosphate to obtain a powder. In the centrifugal fluid coating granule machine, the blank ball core is primed with powder, and the hypromellose aqueous solution is sprayed at the same time. The prepared pill core containing medicine is dried, and wrapped with an isolation layer in a centrifugal fluid coating granulator, and a fluidized bed device is used to prepare an enteric coating layer and a modified coating layer, and finally the pellets are filled into the seaweed polysaccharide capsule shell, The esomeprazole magnesium enteric-coated pellets and capsules are obtained.
Embodiment 2
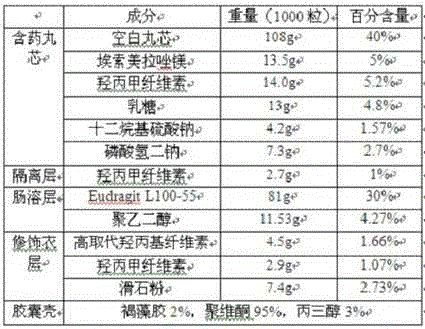
[0179]
[0180] In this example, the blank pellet core is a microcrystalline cellulose pellet core purchased from Haining Weijing Pharmaceutical Excipient Technology Development Co., Ltd.
[0181] Preparation method: dry mix esomeprazole magnesium and part of hydroxypropyl cellulose to obtain powder. In the centrifugal fluid coating granule machine, the blank ball core is primed with powder, and the hydroxypropyl cellulose aqueous solution is sprayed at the same time. The prepared pill core containing medicine is dried, and wrapped with an isolation layer in a centrifugal fluid coating granulator, and a fluidized bed device is used to prepare an enteric coating layer and a modified coating layer, and finally the pellets are filled into the seaweed polysaccharide capsule shell, The esomeprazole magnesium enteric-coated pellets and capsules are obtained.
Embodiment 3
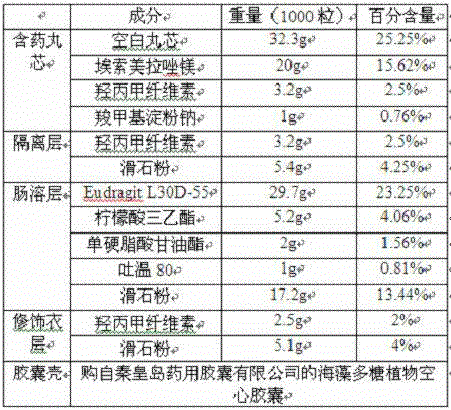
[0183]
[0184] Blank ball core is Suglets in the present embodiment ? CP-405E sucrose pellet core.
[0185] Preparation method: dry mix esomeprazole magnesium with part of sodium carboxymethylcellulose and Tween 80 to obtain a powder. In the centrifugal fluid coating granulator, the blank ball core is primed with powder, and the aqueous solution of sodium carboxymethylcellulose is sprayed at the same time. The prepared pill core containing medicine is dried, and wrapped with an isolation layer in a centrifugal fluid coating granulator, and a fluidized bed device is used to prepare an enteric coating layer and a modified coating layer, and finally the pellets are filled into the seaweed polysaccharide capsule shell, The esomeprazole magnesium enteric-coated pellets and capsules are obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com