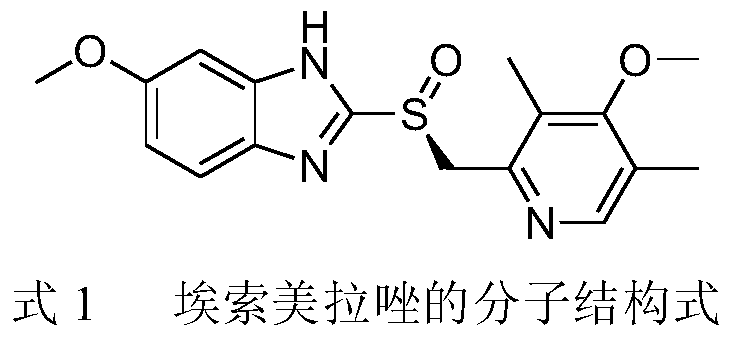
A preparation process and application method of esomeprazole enteric-coated liquid
A technology of esomeprazole sausage and meprazole sausage, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., to avoid degradation and damage, good release and absorption, and good acid resistance force effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Esomeprazole pellets consist of a drug-loaded pellet core, an isolation layer, and an enteric-coated layer, among which:
[0038] Drug-loaded pill core formula:
[0039]
[0040] Preparation method of drug-loaded pellet core: adopt fluidized spray drying technology, spray esomeprazole magnesium in the form of aqueous suspension of binder containing solvent onto sucrose pellet core to form drug-loaded pellet core, wherein the diameter of the pellet core is 0.3 mm, the weight gain of the pellet core is 213%, and the particle size of esomeprazole magnesium is below 5 μm.
[0041] The isolation pellets are:
[0042]
[0043] The preparation method of the isolation layer pellets: the fluidized spray drying technology is adopted, and the drug-loaded pellet core pellets are coated with an isolation layer with a solution containing talcum powder and hypromellose to form isolation layer pellets, and the weight gain of the isolation layer is 24.5%.
[0044] Enteric-coated p...
Embodiment 2
[0054] Esomeprazole pellets consist of a drug-loaded pellet core, an isolation layer, and an enteric-coated layer, among which:
[0055] Drug-loaded pill core formula:
[0056]
[0057] Preparation method of drug-loaded pellet core: adopt fluidized spray drying technology, spray esomeprazole magnesium in the form of aqueous suspension of binder containing solvent onto sucrose pellet core to form drug-loaded pellet core, wherein the diameter of the seed core is 0.3 mm, the weight gain of the seed core is 266.8%, and the particle size of esomeprazole magnesium is below 5 μm.
[0058] The isolation pellets are:
[0059]
[0060] The preparation method of the isolation layer pellets: the fluidized spray drying technology is adopted, and the drug-loaded core pellets are coated with an isolation layer with a solution containing talcum powder and hydroxypropyl cellulose to form isolation layer pellets, and the weight gain of the isolation layer is 22.4%.
[0061] Enteric-coat...
Embodiment 3
[0071] Esomeprazole pellets consist of a drug-loaded pellet core, an isolation layer, and an enteric-coated layer, among which:
[0072] Drug-loaded pill core formula:
[0073]
[0074] Preparation method of drug-loaded pellet core: adopt fluidized spray drying technology, spray esomeprazole magnesium in the form of aqueous suspension of binder containing solvent onto sucrose pellet core to form drug-loaded pellet core, wherein the diameter of the seed core is 0.3 mm, the weight gain of the seed core is 320%, and the particle size of esomeprazole magnesium is below 5 μm.
[0075] The isolation pellets are:
[0076]
[0077] The preparation method of the separation layer pellets: adopt the fluidized spray drying technology, use the hydroxypropyl methylcellulose solution containing titanium dioxide and talcum powder to coat the drug-loaded core pellets with the separation layer to form the separation layer pellets, and the weight gain of the separation layer is 18.8 %. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com