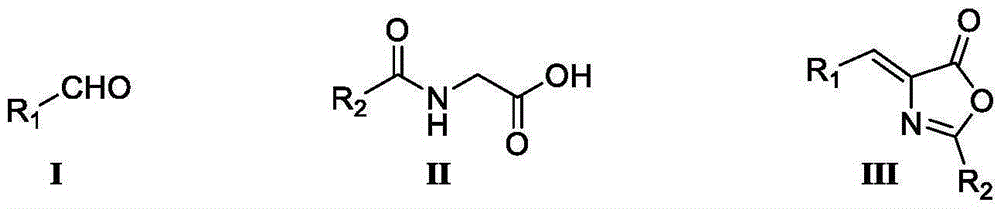
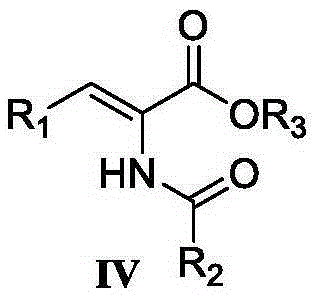
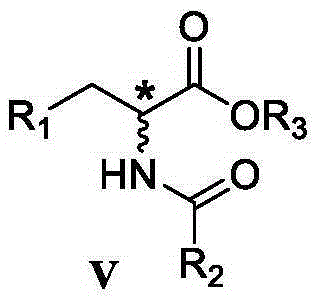
Preparation method of chiral alpha-amino acid
An amino acid and chiral technology, which is applied in the field of preparation of chiral α-amino acids, can solve the problems of increasing the difficulty of process operation, occupying the main cost of the process, and narrow application range, so as to achieve safe, stable and reliable production, high economic benefits and social value , a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: (S)-butanine hydrochloride
[0054] Step 1: (4Z)-Ethylene-2-phenyloxazol-5(4H)-one
[0055] Under nitrogen protection, N-benzoylglycine (0.45mol, 80.6g), anhydrous potassium acetate (0.55mol, 54.0g), acetic anhydride (2mol, 204.0g) were successively dropped into a 1L three-necked flask, and mechanically Stir for 30min. Add acetaldehyde (0.5mol, 22.0g), heat up to 65°C, and keep the temperature of the reaction system at 60-70°C. TLC tracking (developing solvent is n-hexane: ethyl acetate = 10:1) until the raw material point reacted completely. Turn off the heating, cool down to 0-5°C in an ice bath, a large amount of yellow solid precipitates, and stand for 6 hours to crystallize. Suction filtration and vacuum drying yielded a crude yellow solid. The crude product was recrystallized from butyl acetate to obtain 54.2 g of pure product, with a yield of 64.3% and a chemical purity of 99.2%.
[0056] 1 H-NMR (CDCl 3 ,400MHz)δ8.17(d,2H,J=8.0Hz),7.31-7.6...
Embodiment 2
[0066] Embodiment 2: (S)-thienylalanine hydrochloride
[0067] Step 1: (4Z)-thiophenylidene-2-phenyl-oxazol-5(4H)-one
[0068] Under nitrogen protection, N-benzoylglycine (0.55mol, 98.5g), anhydrous sodium acetate (0.5mol, 41.0g), and acetic anhydride (1.5mol, 153.1g) were successively dropped into a 1L three-necked flask. Stir mechanically for 30 min. Add thiophene carboxaldehyde (0.5mol, 56.1g), heat to 95°C, and keep the temperature of the reaction system at 90-100°C. TLC tracking (developing solvent is n-hexane: ethyl acetate = 10:1) until the raw material point reacted completely. Turn off the heating, cool down to 0-5°C in an ice bath, a large amount of yellow solid precipitates, and stand for 6 hours to crystallize. Suction filtration and vacuum drying yielded a crude yellow solid. The crude product was recrystallized from butyl acetate to obtain 110.2 g of the pure product, with a yield of 86.3% and a chemical purity of 99.0%.
[0069] 1 H-NMR (CDCl 3 ,400MHz)...
Embodiment 3
[0079] Example 3: (S)-Phenylalanine Phosphate
[0080] Step 1: (4Z)-Phenylmethylene-2-phenyl-oxazol-5(4H)-one
[0081] Under nitrogen protection, N-benzoylglycine (0.6mol, 107.5g), anhydrous calcium acetate (0.65mol, 102.8g), and acetic anhydride (1.0mol, 102.1g) were successively dropped into a 1L three-necked flask. Stir mechanically for 30 min. Add benzaldehyde (0.5mol, 53.1g), heat to 85°C, and keep the temperature of the reaction system at 80-90°C. TLC tracking (developing solvent is n-hexane: ethyl acetate = 10:1) until the raw material point reacted completely. Turn off the heating, cool down to 0-5°C in an ice bath, a large amount of yellow solid precipitates, and stand for 6 hours to crystallize. Suction filtration and vacuum drying yielded a crude yellow solid. The crude product was recrystallized from butyl acetate to obtain 115.3 g of pure product, with a yield of 92.5% and a chemical purity of 99.3%.
[0082] 1 H-NMR (CDCl 3 ,400MHz)δ8.16(d,2H,J=8.0Hz),7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com