Asymmetric-structure modified epoxy acrylic resin and continuous method synthetic method
An epoxy acrylate, asymmetric structure technology, applied in the direction of epoxy resin coating, application, coating, etc., can solve the problems of high molecular symmetry, poor surface dryness, gloss reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
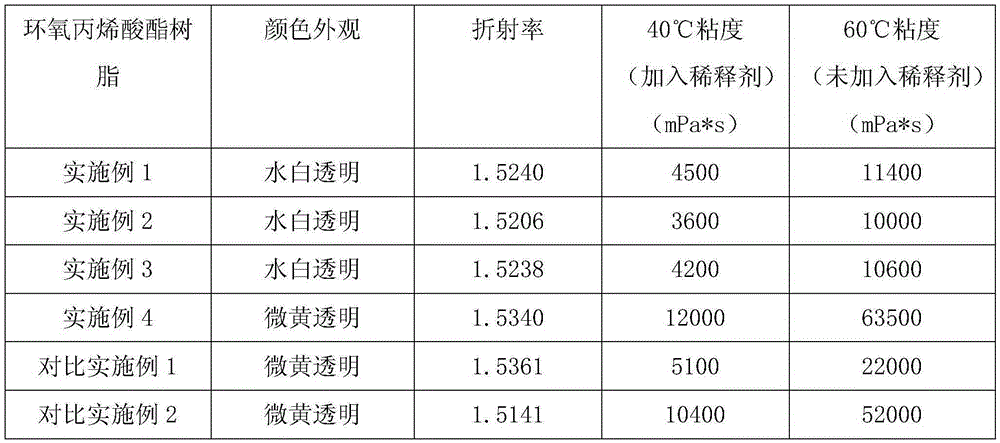
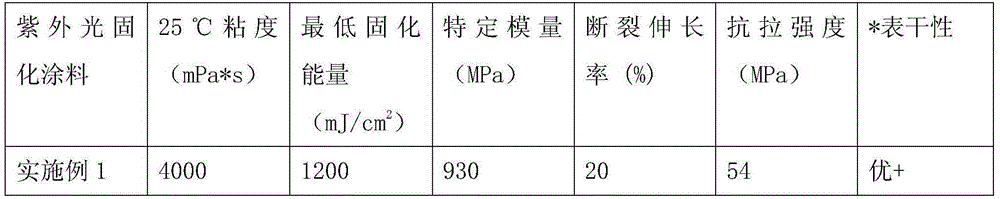
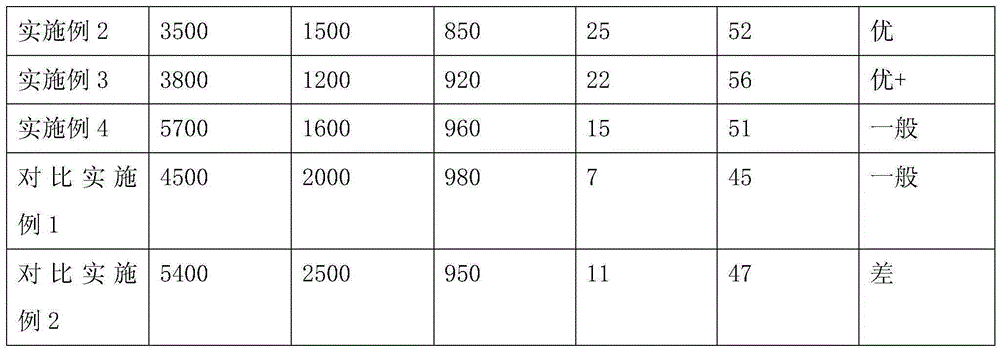
Embodiment 1
[0066] Add 250.0g of epoxy resin E-51 and 0.52g of antioxidant Irganox1010 into a 500ml four-necked flask equipped with a thermometer, a mechanical stirrer and a condenser, raise the temperature to 90°C while stirring at 200-250rpm, add dropwise a mixture of 49.10g The liquid of acrylic acid, 1.26g triphenylphosphine and 0.30g p-hydroxyanisole, and control the rate of addition dropwise within 1h, then raise the temperature to 110°C to continue the reaction for 4h, take samples every half hour to monitor the acid value, when When it is less than 5mgKOH / g, lower the temperature to 75°C, add 69.60g of allyl hydroxyethyl ether, 101.04g of phthalic anhydride and 1.26g of triphenylphosphine at one time, stir and react at 80°C at 200-250rpm for 3h, then lower the temperature Raise to 110°C, continue the reaction for 4 hours, take samples every half hour to monitor the acid value, when it is less than 5mgKOH / g, lower the temperature to 90°C, add 95.31g of tripropylene glycol diacrylate...
Embodiment 2
[0069] Add 200.40g of epoxy resin E-51 and 0.52g of antioxidant Irganox1010 into a 500ml four-neck flask equipped with a thermometer, mechanical stirring and condenser, heat up to 90°C while stirring at 200-250rpm, and add 20.85g of The liquid of acrylic acid, 1.26g triethylamine and 0.32g p-hydroxyanisole, and control the rate of addition dropwise within 1h, then raise the temperature to 110°C to continue the reaction for 4h, take samples every half hour to monitor the acid value, when less than When the temperature is 5mgKOH / g, cool down to 75°C, add 112.25g allyl hydroxyethyl ether, 161.70g phthalic anhydride and 1.26g triethylamine at one time, stir and react at 80°C at 200-250rpm for 3h, then raise the temperature to 110°C, continue to react for 4 hours, take samples every half hour to monitor the acid value, when it is less than 5mgKOH / g, cool down to 90°C, add 95.31g of tripropylene glycol diacrylate, stir at 300-350rpm for 10min, and then discharge.
[0070] Various pe...
Embodiment 3
[0072] Add 200.40g of epoxy resin E-51 and 0.52g of antioxidant Irganox1010 into a 500ml four-neck flask equipped with a thermometer, mechanical stirring and condenser, heat up to 90°C while stirring at 200-250rpm, add dropwise a mixture of 40.92g The liquid of acrylic acid, 1.26g triethylamine and 0.32g p-hydroxyanisole, and control the rate of addition dropwise within 1h, then raise the temperature to 110°C to continue the reaction for 4h, take samples every half hour to monitor the acid value, when less than When the temperature is 5mgKOH / g, lower the temperature to 90°C, add 55.64g of allyl hydroxyethyl ether, 84.22g of hexahydrophthalic anhydride and 1.26g of triethylamine at one time, stir and react at 95°C at 200-250rpm for 3h, then lower the temperature Raise to 110°C, continue the reaction for 4 hours, take samples every half hour to monitor the acid value, when it is less than 5mgKOH / g, lower the temperature to 90°C, add 95.31g of tripropylene glycol diacrylate, stir ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com