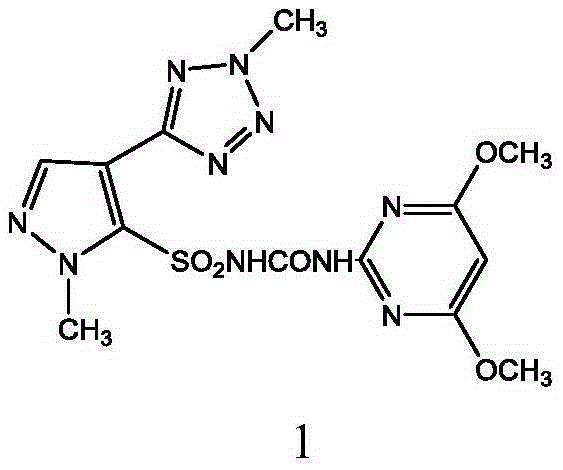
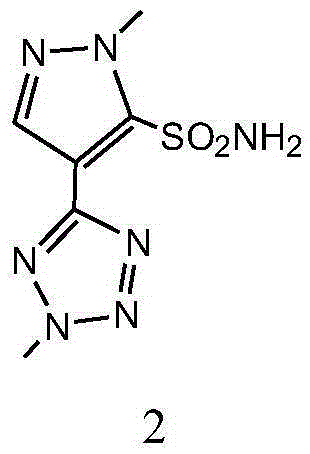
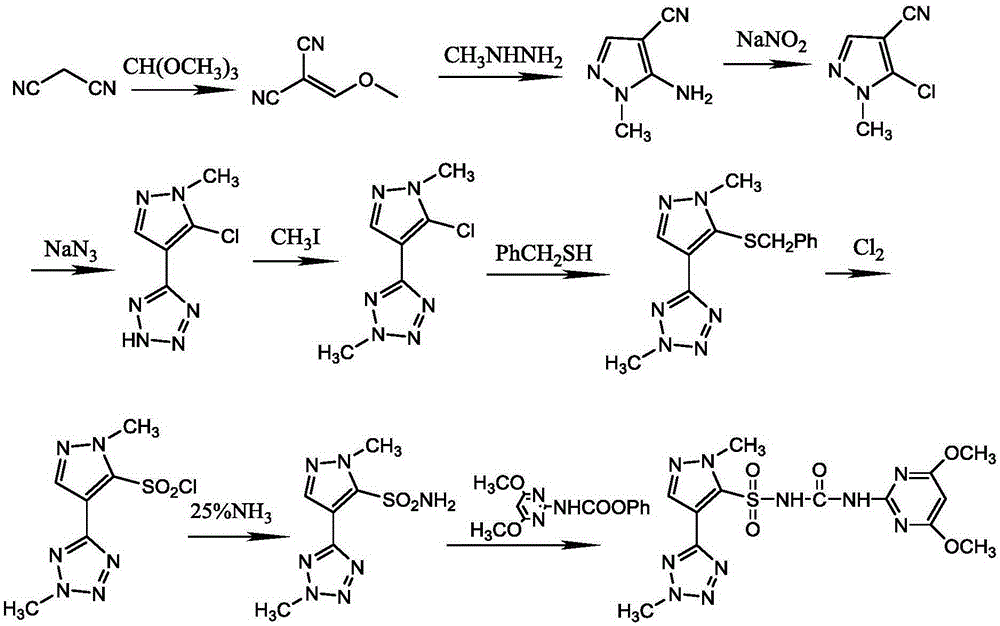
Preparation method and applications of azimsulfuron key intermediate
A technology of tetrazole and pyrazole, applied in the new preparation, the application field in the preparation of rimsulfuron-methyl tetrazole, can solve the problems of adverse environmental impact, long route, environmental pollution, etc., and achieve the goal of being suitable for industrial production and improving reaction Efficiency, the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of 1-methyl-4-(2-methyl-2H-tetrazol-5-yl)-1H-pyrazole-5-sulfonamide
[0059] Step 1, Synthesis of 2-Methoxymethenylmalononitrile
[0060]
[0061] Add 53g (0.5mol) trimethyl orthoformate, 33g (0.5mol) malononitrile, and 200mL acetic anhydride into a 500mL reaction flask equipped with a reflux tube and a thermometer. Under electric stirring, heat to reflux for 6h, then Distill out low-boiling solvents such as acetic anhydride and by-product methyl acetate. Cool to room temperature (solids are precipitated at this time), add appropriate amount of methanol, wait until the solids are completely dissolved, add activated carbon for decolorization, filter while hot, freeze the filtrate in the refrigerator for 1-3h, precipitate solids, filter with suction, and wash the filter cake with methanol. After drying, 41.04 g of the title compound (white solid) was obtained with a yield of 67.4%.
[0062] m / z:108[M+1] + .
[0063] Step 2. Synthesis of 1-methyl-4-cyano-5-...
Embodiment 2
[0083] Example 2 Preparation of 1-methyl-4-(2-methyl-2H-tetrazol-5-yl)-1H-pyrazole-5-sulfonamide
[0084] Step 1, Synthesis of 2-Methoxymethenylmalononitrile
[0085]
[0086] Add 53 g (0.5 mol) trimethyl orthoformate, 33 g (0.5 mol) malononitrile, and 200 mL acetic anhydride into a 500 mL reaction flask equipped with a reflux tube and a thermometer. Under electric stirring, heat and reflux for 10 hours, then Distill out low-boiling solvents such as acetic anhydride and by-product methyl acetate. Cool to room temperature (solids are precipitated at this time), add appropriate amount of methanol, wait until the solids are completely dissolved, add activated carbon for decolorization, filter while hot, freeze the filtrate in the refrigerator for 1-3h, precipitate solids, filter with suction, and wash the filter cake with methanol. After drying, 42 g of the title compound (white solid) was obtained with a yield of 69%
[0087] m / z:108[M+1] + .
[0088] Step 2. Synthesis of 1-methyl-4...
Embodiment 3 4
[0108] Example 3 Synthesis of Tetrazosulfuron
[0109]
[0110] 60.5 g (0.25 mol) of 1-methyl-4-(2-methyl-2H-tetrazol-5-yl)-1H-pyrazole-5-sulfonamide prepared according to the method described in Example 2 , (4,6-Dimethoxypyrimidin-2-yl) phenylcarbamate 68.7g (0.25mol), potassium carbonate 55.2g (0.4mol) and 200ml dioxane were put into the reaction flask, stirred to dissolve, Then stir at 40-50°C for 5h. Most of the solvent was evaporated under reduced pressure, 200ml of water was added, neutralized with hydrochloric acid to neutral pH, filtered, the filter cake was washed with water, and dried to obtain 99.4 g of the title compound with a yield of 93.3%. Melting point: 173-176, HPLC purity 98.6%.
[0111] m / z: 421.
[0112] 1 H-NMR(CDCl3): δ2.50(s, CH 3 ), 2.51(s, CH 3 ),3.81(s, 2CH 3 O), 4.67 (s, CH), 5.80 (s, CH-Pyrimidin).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com