Synthesizing method for 9,10-dimethylanthracene
A technology of dimethyl anthracene and synthetic method, which is applied in 9 fields, and achieves the effects of low preparation cost, less by-products and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
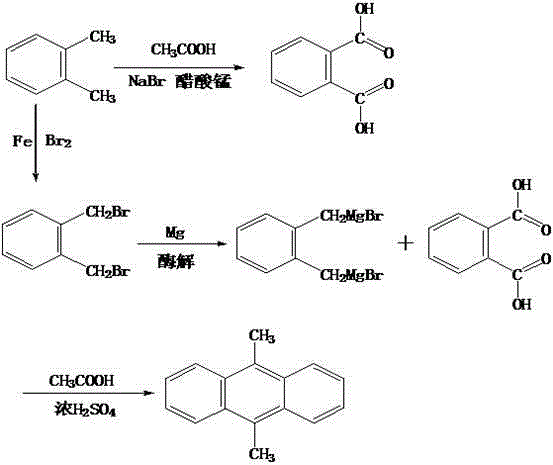
[0015] Firstly, put 30mL of o-xylene into the reaction kettle, add 0.5g of manganese acetate, 15mL of acetic acid and 10mL of paraldehyde into it respectively, and react for 2 hours at a temperature of 210°C and a pressure of 2.5MPa. After the reaction is completed, , placed in a centrifuge for centrifugal separation, and dried to obtain crude phthalic acid; then, under the conditions of controlling the temperature at 280°C and the pressure at 6.5MPa, dissolve the crude phthalic acid obtained above in 30mL of water, and then add 1g of sodium bromide , pass through hydrogen to react for 1 hour, after the reaction is completed, place in ice water for 8 minutes, filter, wash once with water, dry to obtain fine phthalic acid, and set aside; then take 30mL of o-xylene into the reaction container, pour it into Add 10mL of liquid bromine and 1g of iron powder respectively, control the temperature at 350°C and rotate at 700r / min, stir magnetically for 40min, after the stirring is compl...
example 2
[0017] Firstly, put 32mL o-xylene into the reaction kettle, add 0.7g manganese acetate, 18mL acetic acid and 12mL paraldehyde to it, and react for 2.5h at a temperature of 220°C and a pressure of 2.8MPa until the reaction is complete Afterwards, put it in a centrifuge for centrifugation and dry to obtain the crude phthalic acid; then, under the conditions of controlling the temperature at 285°C and the pressure at 6.7MPa, dissolve the crude phthalic acid obtained above in 35mL of water, and then add 1.1g of bromine Sodium chloride, add hydrogen to react for 1.5h, after the reaction is completed, place in ice water for 9min, filter, wash twice with water, dry to obtain fine phthalic acid, set aside; then take 32mL o-xylene and put it into the reaction container , add 13mL of liquid bromine and 1.5g of iron powder to it respectively, control the temperature at 360°C and rotate at 800r / min, stir magnetically for 45min, after the stirring is completed, cool down to 75°C at a speed ...
example 3
[0020]Firstly, put 35mL of o-xylene into the reaction kettle, add 0.9g of manganese acetate, 20mL of acetic acid and 14mL of paraldehyde respectively, and react for 3 hours at a temperature of 230°C and a pressure of 3.0MPa. After the reaction is completed, , placed in a centrifuge for centrifugal separation, and dried to obtain crude phthalic acid; then, under the conditions of controlling the temperature at 290°C and the pressure at 6.9MPa, dissolve the crude phthalic acid obtained above in 40mL of water, and then add 1.2g bromide sodium, and hydrogen gas was introduced to react for 2 hours. After the reaction was completed, put it in ice water for 10 minutes, filter it, wash it twice with clean water, and dry it to obtain fine phthalic acid. Add 15mL of liquid bromine and 2g of iron powder respectively, control the temperature at 370°C and rotate at 900r / min, stir magnetically for 50min, after the stirring is completed, cool down to 80°C at a speed of 15°C / min, react for 2h,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com