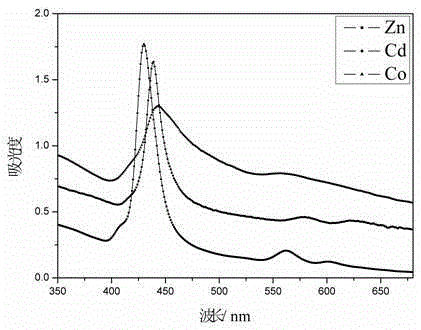
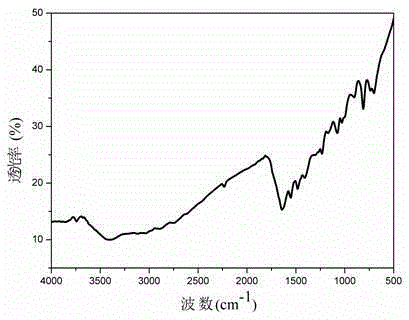
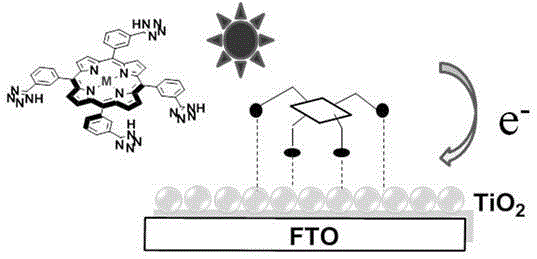
Tetrazole porphyrin metal complexes, synthesis thereof and applications of the metal complexes as dye sensitizing agents
A technology of tetrazolium porphyrin and metal complexes, which can be used in organic chemistry, photosensitive equipment, electrolytic capacitors, etc., can solve the problems of ruthenium complex consumption, pollution, production restrictions, etc., and achieves the promotion of photoelectric conversion efficiency, and the raw materials are simple and easy. The effect of obtaining, good photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Synthesis of phenyl zinc porphyrin——5,10,15,20-tetrakis(3-tetrazolyl)phenyl zinc porphyrin
[0039] (1) Add freshly distilled pyrrole (7.5mmol, 0.52mL) and 3-cyanobenzaldehyde (7.5mmol, 0.98g) into 50mL of propionic acid, reflux at 145~150℃ for 1.5~2h, after the end of the reaction, lower the temperature Reduce to 90~100°C, distill off propionic acid under reduced pressure, evaporate to dryness, dissolve with chloroform, add 5% sodium bicarbonate solution to adjust pH to alkaline, extract with chloroform-5% sodium bicarbonate, collect The organic layer was dried with anhydrous sodium sulfate, filtered, and the reaction solution was concentrated by rotary evaporation to obtain a crude product; the crude product was placed on the top of neutral alumina (diameter 5cm×length 15cm), eluted with dichloromethane, and the first layer of purple was collected. The red layer was evaporated to dryness to obtain the purple powder product 5,10,15,20-tetrakis(3-cyano)phenylporphyri...
Embodiment 2
[0050] 1. Synthesis of phenylcadmium porphyrin——5,10,15,20-tetrakis(3-tetrazolyl)phenylcadmium porphyrin
[0051] (1), (2) are the same as embodiment one;
[0052] (3) Dissolve 5,10,15,20-tetrakis(3-tetrazolyl)phenylporphyrin (0.4mmol, 0.35g) in anhydrous DMF (20mL), add Cd(OAc) 2 2H 2 O (2mmol, 0.533g), refluxed for 4~5 hours, cooled to room temperature, evaporated to dry DMF, washed several times with water-dichloromethane solution to obtain the product 5,10,15,20-tetrakis(3-tetrazolyl) The crude product of phenyl cadmium porphyrin, the crude product was separated by silica gel column chromatography to obtain the final product of dark green powder, the yield: 58%.
[0053] Its structure is as follows:
[0054]
[0055] 2. Preparation of the dye-sensitized solar cell: the preparation method is the same as in Example 1.
[0056] The performance of the battery: the curve of the relationship between the current density and the voltage of the dye-sensitized solar cell is s...
Embodiment 3
[0058] 1. Synthesis of phenylcobalt porphyrin - 5,10,15,20-tetrakis(3-tetrazolyl)phenylcobalt porphyrin
[0059] (1), (2) are the same as embodiment one;
[0060] (3) Dissolve 5,10,15,20-tetrakis(3-tetrazolyl)phenylporphyrin (0.4mmol, 0.35g) in anhydrous DMF (20mL), add Co(OAc) 2 2H 2 O (2mmol, 0.498g), refluxed for 4~5 hours, cooled to room temperature, evaporated to dry DMF, washed several times with water-dichloromethane solution to obtain the product 5,10,15,20-tetrakis(3-tetrazolyl) The crude product of phenylcobalt porphyrin was separated by silica gel column chromatography to obtain the final product as a dark green powder with a yield of 53%.
[0061] Its structure is as follows:
[0062]
[0063] 2. Preparation of the dye-sensitized solar cell: the preparation process is the same as in Example 1.
[0064] Performance of Dye-Sensitized Solar Cells: Curves of Current Density vs. Voltage See Figure 4 : J is 0.435mA / cm 2 , V is 363mV, and the maximum value of IP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com