Preparation method for rosuvastatin calcium intermediate
A technology for rosuvastatin calcium and intermediates, which is applied in the field of compound synthesis technology, can solve the problems of poor reaction selectivity, large amount of waste water, and high production cost, and achieve the effects of mild reaction conditions, environmental friendliness, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
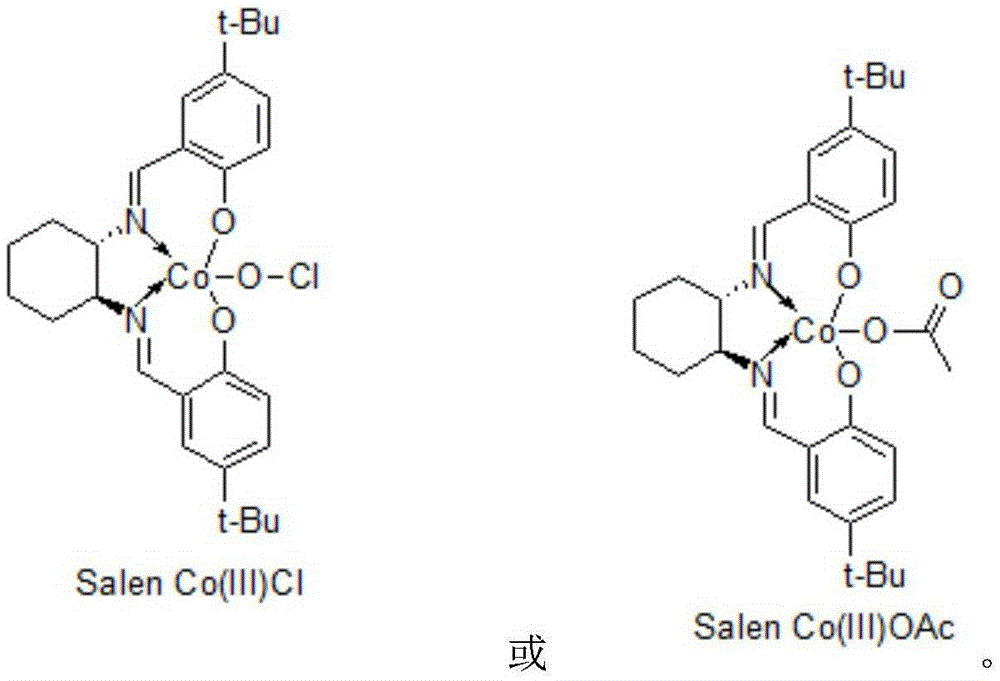
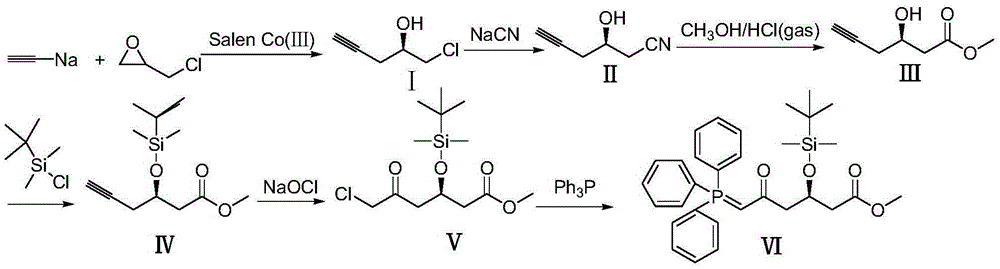
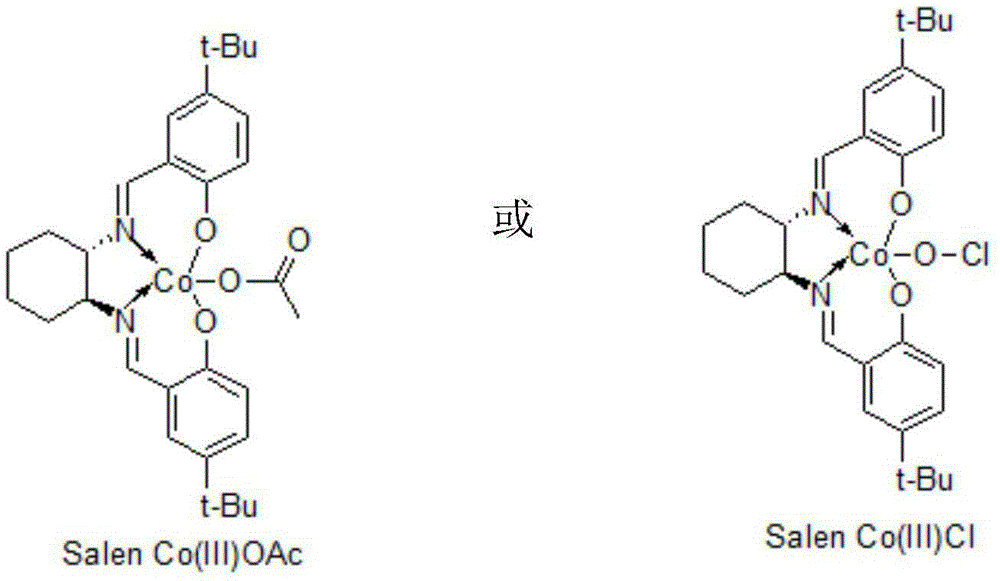
[0012] Add 0.275 g of SalenCo(III) (OAc) to 160 g of 30% sodium acetylene tetrahydrofuran solution, dropwise add 92.0 g of epichlorohydrin, react for 12 hours, concentrate and recover THF, add 200 ml of ethyl acetate, and wash with 100 ml of saturated saline , layered, concentrated and recovered ethyl acetate to obtain (2R)-1-chloro-2-hydroxyl-4-pentyne (I) 98.0 grams; 118.0 grams of (2R)-1-chloro-2-hydroxyl-4-pentyne Alkyne (I) was added dropwise in 245 grams of 20% sodium cyanide aqueous solution, and reacted for 4 hours at 30°C. After the reaction was completed, 500 ml of ethyl acetate was added, followed by washing with 100 ml of saturated brine, layering, concentration and recovery of ethyl acetate to obtain ( 2R)-1-cyano-2-hydroxy-4-pentyne (II) 98.0 g. 109.0 grams of (2R)-1-cyano-2-hydroxyl-4-pentyne (II) was added dropwise in 1000ml of methanol, followed by hydrogen chloride gas and gas chromatography to track the end of the reaction, and reacted at 0°C for 10 hours. A...
Embodiment 2
[0014] In 160 grams of 30% sodium acetylene tetrahydrofuran solution, add 0.542 grams of SalenCo(Ⅲ) Cl, dropwise add 101.2 grams of epichlorohydrin, react for 24 hours, concentrate and recover tetrahydrofuran, add 200 ml of ethyl acetate, add 100 ml of saturated saline to wash, divide layer, concentrated and recovered ethyl acetate to obtain (2R)-1-chloro-2-hydroxyl-4-pentyne (I) 100.2 grams; 118.0 grams of (2R)-1-chloro-2-hydroxyl-4-pentyne ( 1) Add dropwise to 257.3 grams of 20% sodium cyanide aqueous solution, and react at 30°C for 8 hours. After the reaction, add 500 ml of ethyl acetate, add 100 ml of saturated saline to wash, separate layers, concentrate and recover ethyl acetate to obtain (2R) - 98.9 g of 1-cyano-2-hydroxy-4-pentyne (II). 109.0 grams of (2R)-1-cyano-2-hydroxyl-4-pentyne (II) was added dropwise to 1000ml of methanol, followed by hydrogen chloride gas, followed by gas chromatography at the end of the reaction, and reacted at 5°C for 5 hours. After the reac...
Embodiment 3
[0016]Add 0.50 g of SalenCo(Ⅲ)Cl to 160 g of 30% sodium acetylene tetrahydrofuran solution, add dropwise 100.0 g of epichlorohydrin, react for 18 hours, concentrate and recover tetrahydrofuran, add 200 ml of ethyl acetate, add 100 ml of saturated saline to wash, divide layer, concentrated and recovered ethyl acetate to obtain (2R)-1-chloro-2-hydroxyl-4-pentyne (I) 112.2 grams; 118.0 grams of (2R)-1-chloro-2-hydroxyl-4-pentyne ( 1) Add dropwise to 250 grams of 20% sodium cyanide aqueous solution, and react at 30°C for 8 hours. After the reaction, add 500 ml of ethyl acetate, add 100 ml of saturated saline to wash, separate layers, concentrate and recover ethyl acetate to obtain (2R) - 100.1 g of 1-cyano-2-hydroxy-4-pentyne (II). 109.0 grams of (2R)-1-cyano-2-hydroxyl-4-pentyne (II) was added dropwise to 1000ml of methanol, followed by hydrogen chloride gas, followed by gas chromatography at the end of the reaction, and reacted at 0°C for 8 hours. After the reaction, Methanol w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com