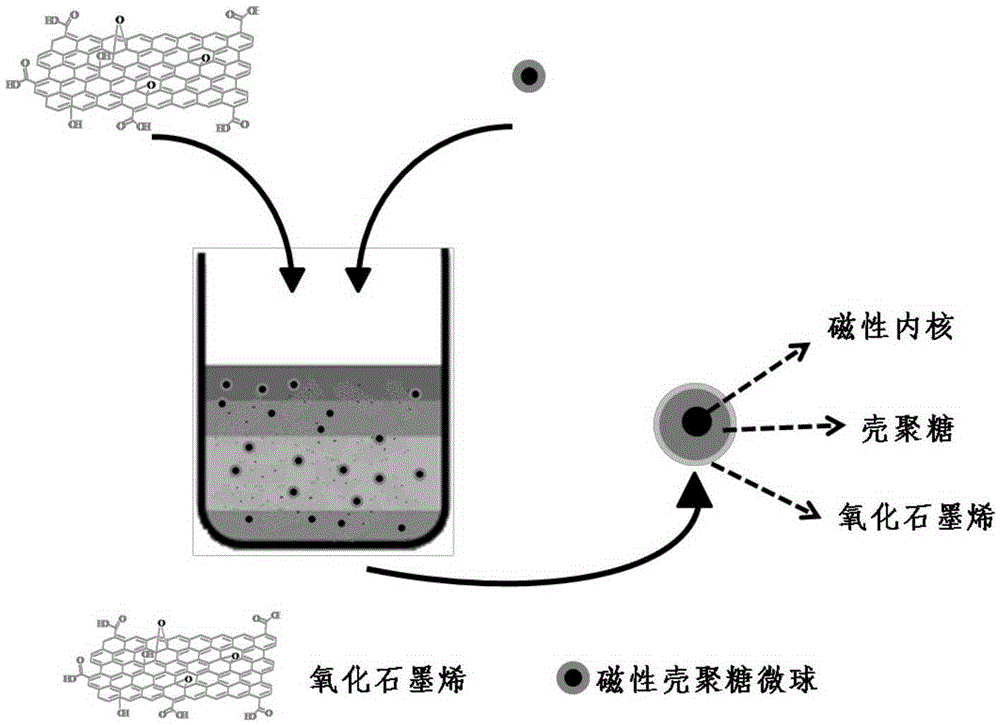
Method for preparing functionalized graphene
A technology of fossil and graphene, applied in the field of preparation of functionalized graphene, can solve problems such as high price and limited application, and achieve the effects of simple preparation process, wide source and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Preparation of graphene oxide: Add 1g of natural graphite powder (8000 mesh) and 30mL of concentrated sulfuric acid into a single-necked round-bottomed flask, slowly magnetically stir for 6h under cooling in a dry ice bath, and then slowly add 3gKMnO under vigorous stirring. 4 Powder, react at 0-20°C for 30 minutes, then react at 33-35°C for 30 minutes. Subsequently, 30 mL of deionized water was slowly added and reacted at 93-95° C. for 35 min. Finally, 140 mL of deionized water and 10 mL of 30% hydrogen peroxide solution were added to terminate the reaction. After the reaction, the mixed solution was filtered with a microporous membrane (pore size 4 2- ), use barium chloride solution to detect whether the excess SO 4 2- remove. Finally, the material prepared by the above method was vacuum-dried at 60° C. to obtain graphite oxide. Weigh a certain amount of graphite oxide and disperse it in deionized water, after ultrasonic stripping for 3-5 hours, centrifuge at ...
Embodiment 2
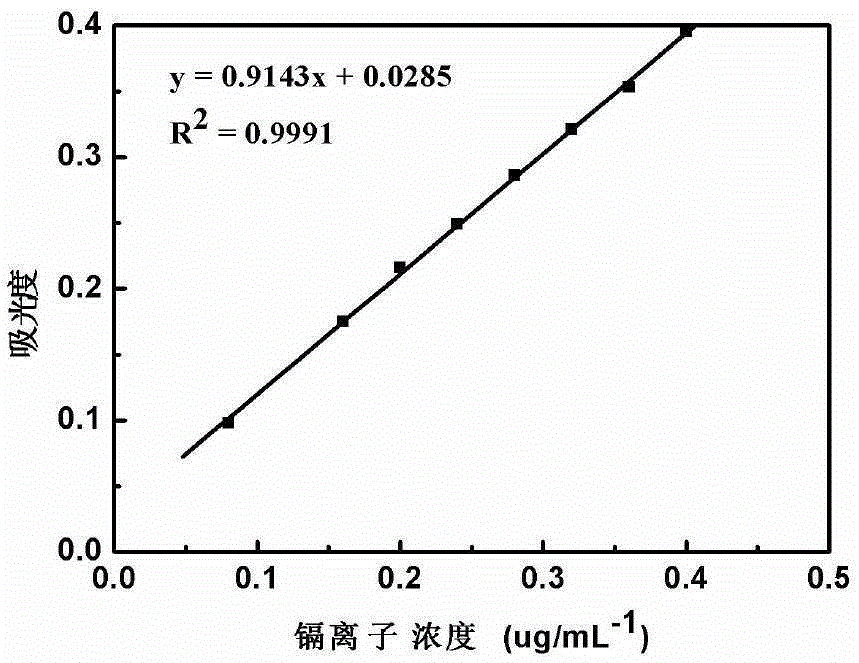
[0046] Take 5.0 g of functionalized graphene, and take 20.00 mL of mass concentration as 10 μg·mL -1 Cd 2+ As the research object, when the adsorption time is 3.5h, the pH is 5.0, and the adsorption temperature is 35℃, the adsorption capacity of the solution is the best. The unit adsorption capacity is 136.07μg·g -1, The adsorption rate can reach 75.85%. Its adsorption kinetics conforms to the Freundlich formula. According to thermogravimetric analysis, the functionalized graphene can exist stably at 150°C, and has good thermal stability. And due to the existence of magnetic chitosan microspheres, magnetic separation can be realized.
[0047] Functionalized graphene to Cd under optimal conditions in the present embodiment of table 1 2+ Adsorption
[0048]
Embodiment 3
[0050] Get functionalized graphene 5.0g, to 20mL mass concentration be the Cu of 100mg / L 2+ The adsorption test of the solution was carried out. When the adsorption time was 4h, the pH was 6.0, and the adsorption temperature was 30°C, the Cu 2+ The adsorption capacity of ions is the best. The unit adsorption capacity is 1.71mg / g, and the adsorption rate can reach more than 99%. Its adsorption kinetics conforms to the Langmuir formula. According to thermogravimetric analysis, the functionalized graphene can exist stably at 150°C, and has good thermal stability. And due to the existence of magnetic chitosan microspheres, magnetic separation can be realized.
[0051] Functionalized graphene to Cu under optimal conditions in the present embodiment of table 2 2+ Adsorption
[0052]
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com