Synthesis method of cis-7-tetradecenol acetate, the main component of sex pheromone of Ulmus beetle moth
A technology of carbenol acetate and tetradecenol, which is applied in the field of synthesis of acetates, can solve problems such as low yield, high synthesis cost, and dangerous production process, and achieve high product yield and easy operation , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
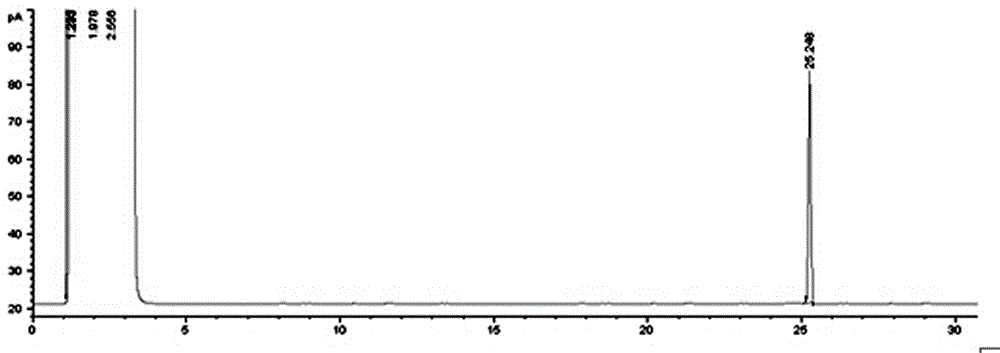
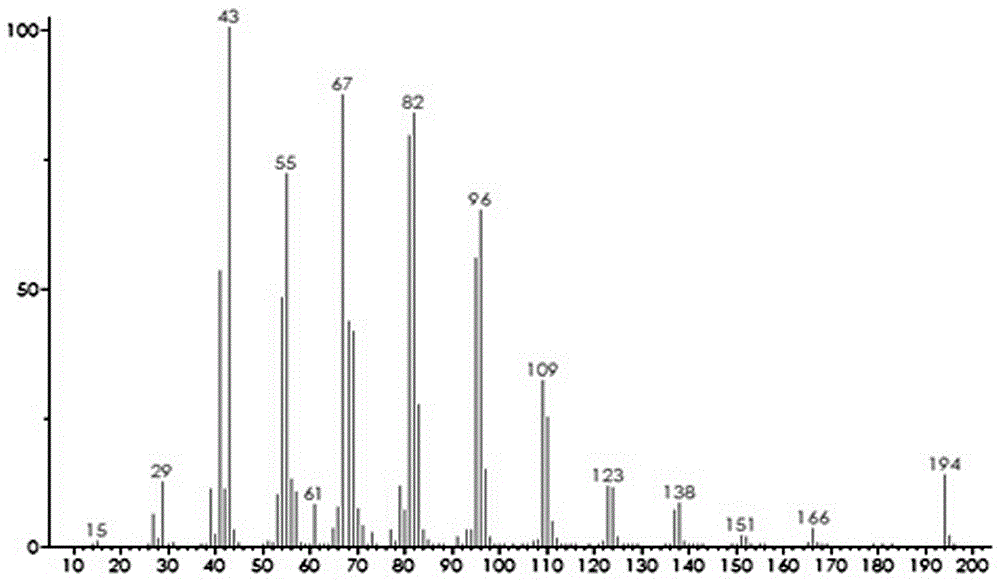
Image
Examples
Embodiment 1
[0017] A method for synthesizing cis-7-tetradecenyl acetate, the main component of the sex pheromone of the elm beetle moth, comprises the following steps:
[0018] (1) Preparation of (7-bromoheptyl-1-)-2-tetrahydropyranyl ether In a 250 mL four-necked flask equipped with an electromagnetic stirrer, a reflux condenser, and a constant pressure dropping funnel, add 3 , 8 g of 4-dihydropyran, add 0.03 g of 37% hydrochloric acid as a catalyst under stirring, start to drop 17 g of 7-bromo-1-heptanol after 10 min, and control the rate of addition (this reaction is an exothermic reaction , when the drop rate of 7-bromo-1-heptanol is accelerated, the reaction will be accelerated and the exotherm will increase, and it must be cooled in an ice bath), keep the temperature of the reaction system at 40-60°C, react at room temperature for 2 hours after the addition, stop stirring, Change to a distillation device to evaporate unreacted 3, 4-dihydropyran. After the residual liquid was cooled...
Embodiment 2
[0024] (1) Preparation of (7-bromoheptyl-1-)-2-tetrahydropyranyl ether In a 500 mL four-neck flask equipped with an electromagnetic stirrer, a reflux condenser, and a constant pressure dropping funnel, add 3 , 20 g of 4-dihydropyran, under stirring, add 0.06 g of 37% hydrochloric acid as a catalyst, start to drop 34 g of 7-bromo-1-heptanol after 10 min, and control the rate of addition (this reaction is an exothermic reaction , when the drop rate of 7-bromo-1-heptanol is accelerated, the reaction will be accelerated and the exotherm will increase, and it must be cooled in an ice bath), keep the temperature of the reaction system at 40-60°C, react at room temperature for 2 hours after the addition, stop stirring, Change to a distillation device to evaporate unreacted 3, 4-dihydropyran. After the residual liquid was cooled, 170 g of petroleum ether was added, washed with saturated sodium bicarbonate solution and saturated brine, dried over sodium sulfate for 10-15 h, concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com