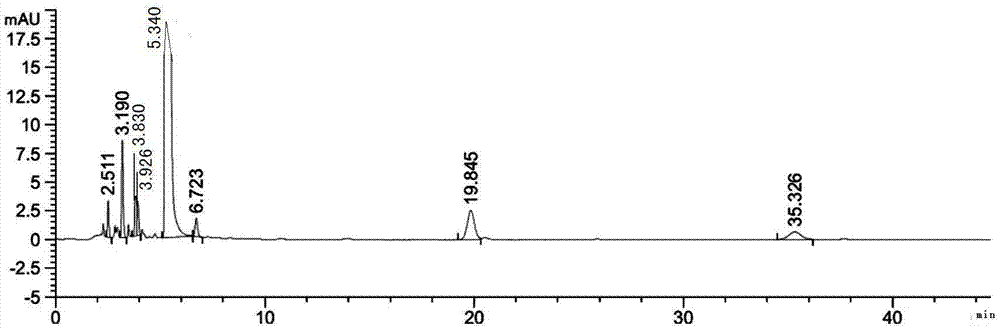
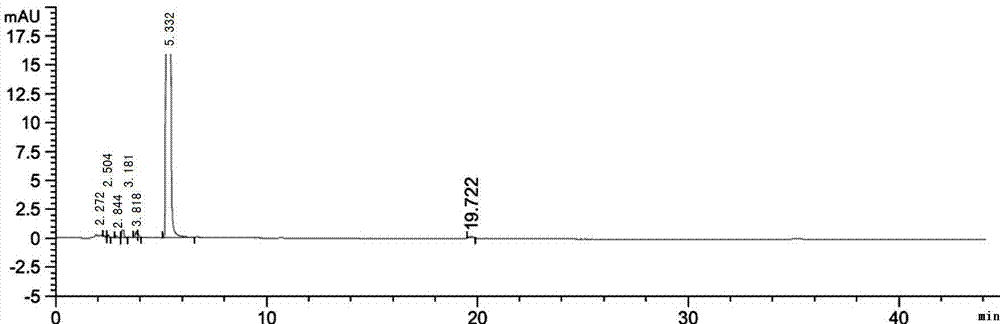
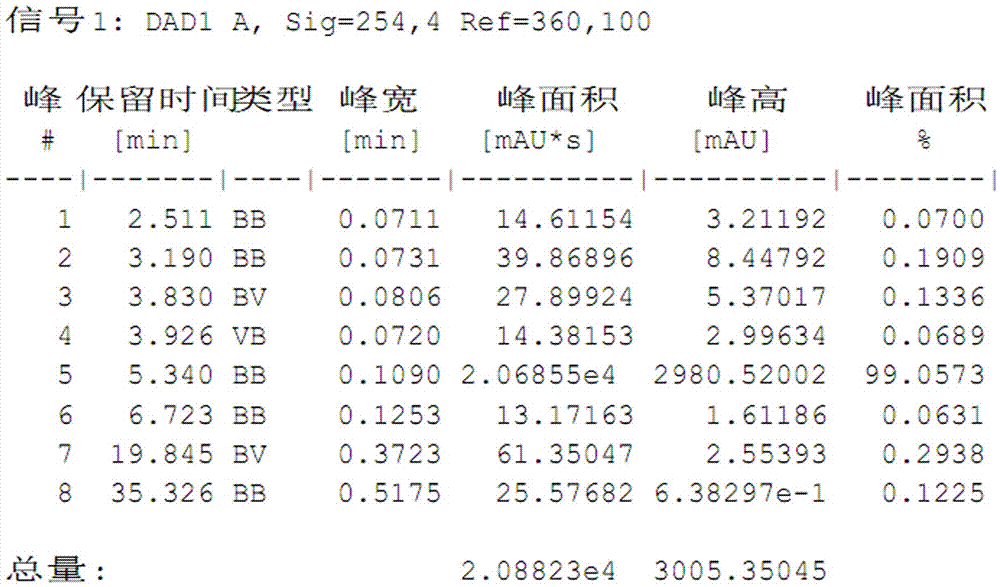
Process for preparing high-purity cefathiamidine
A cefathiamidine, high-purity technology, applied in the field of chemical drug synthesis, can solve the problem of high residual impurities, achieve the effects of high reaction yield, avoid side reactions, and simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0040]In a 10L reaction kettle, add DCM5320g (4L), TEA (200mL), bromoacetyl-7ACA500g in sequence, stir to dissolve, add co-solvent pure water 50mL, then add N,N-diisopropylthiourea 225g, stir to dissolve After clearing, heat the reaction solution to reflux. After 2-3 hours of reaction, stop the reaction and lower the internal temperature to 0°C to 15°C. Add 2L of acetone dropwise, stir and crystallize. After the dropwise addition, keep stirring at 8-12°C and filter with suction. After the filter cake is drained, add 1L of acetone to rinse the filter cake. After draining, add the filter cake to 10L Then add 600mL of pure water and stir to dissolve, then add 1080g (1.2L) of ethyl acetate, stir for 10 minutes, extract impurities, after standing for stratification, filter the water layer into the crystallization reaction kettle, wash with dilute hydrochloric acid Adjust the pH of the solution to 5.0, cool the obtained clear mixed solution to 0-5°C, add 5.6L of acetone dropwise, af...
Embodiment 3
[0042] Add 400mL of DCM, 18mL of TEA, and 50g of bromoacetyl-7ACA to a 1L reaction bottle in turn, stir to dissolve, add 10mL of co-solvent pure water, then add 22.5g of N,N-diisopropylthiourea, stir to dissolve, then heat the reaction solution Return to reflux, react for 2-3 hours, stop the reaction, add 10 mL of pure water as a co-solvent, and lower the internal temperature to 10°C to 15°C. Add 200mL of acetone dropwise, stir and crystallize. After the dropwise addition, keep stirring at 8-12°C and filter with suction. After the filter cake is drained, add 100mL of acetone to rinse the filter cake. In the reaction flask, add 60mL of pure water and stir to dissolve, then add 108g (120mL) of ethyl acetate, stir for ten minutes, extract impurities, stand and separate the layers, filter the water layer into the crystallization reaction flask, adjust with dilute hydrochloric acid Solution pH=4.5, the obtained clear mixed solution was cooled to 0-5°C, and 560mL of acetone was adde...
Embodiment 4
[0044] Add 400mL of DCM, 20mL of TEA, and 50g of bromoacetyl-7ACA to a 1L reaction flask in sequence, stir to dissolve, then add 22.5g of N,N-diisopropylthiourea, stir to dissolve, then heat the reaction solution to reflux, Reaction 2-3 After one hour, the reaction was stopped, and the internal temperature was lowered to 10°C to 15°C. Add 200mL of acetone dropwise, stir and crystallize. After the dropwise addition, keep stirring at 8-12°C and filter with suction. After the filter cake is drained, add 100mL of acetone to rinse the filter cake. In the reaction flask, add 60mL of pure water and stir to dissolve, then add 108g (120mL) of ethyl acetate, stir for ten minutes, extract impurities, stand and separate the layers, filter the water layer into the crystallization reaction flask, adjust with dilute hydrochloric acid Solution pH=5.0, the obtained clear mixed solution was cooled to 0-5°C, and 560mL of acetone was added dropwise. cake. After the filter cake was drained, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com