Monoamine oxidase and application thereof in synthesis of chiral azabicyclic compounds
A technology for monoamine oxidase and oxidase activity, applied in the field of bioengineering, can solve the problems of low corresponding selectivity, incomplete reaction, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The enzyme activity assay of embodiment 1 monoamine oxidase
[0077] Dissolve 5mmol / L benzylamine in 50mmol / L phosphate buffer to obtain a substrate solution, take 1mL and add 10μL enzyme solution, react at 30°C for 20min, add 400μL dinitrophenylhydrazine (1mmol / L, dissolved in 3M HCl), and then add 2.4mL1.25MNaOH, react for 5min, and detect the absorbance at a wavelength of 450nm.
[0078]Optical purity: Agilent1260 series HPLC, chiral column is ChiralpakAD-H (4.6×250mm, Diacel ChemicalInd.Ltd.), analyzed at 25°C, mobile phase is 90% n-heptane, 10% ethanol, 0.1% trifluoroacetic acid, flow rate It is 1mL / min, and the detection wavelength is 220nm. The retention time of the S-configuration product was 7.4min, and the retention time of the R-configuration product was 8.9min.
Embodiment 2
[0079] Embodiment 2 screening monoamine oxidase
[0080] Soil sample DNA (ChromaSpinTE-1000, Clontech Laboratories, Inc.USA) was collected from the campus of Wuhan University, partially digested with Sau3AI, and 1-4kb fragments were collected by electrophoresis, recovered and connected to the BamHI site of pUC19 to obtain a plasmid library; Transform the library into E.coliDH10b, smear it on an LB plate containing 100 μg / mL ampicillin, select positive clones and transfer it to a 96 deep-well plate with 500 μg / mL LB (100 μg / mL ampicillin), culture at 37°C for 4 hours, then add 1 mmol / LIPTG Induction, continue culturing overnight at 30°C, then transfer 10 μL of deep well culture to a new 96-well plate containing 50mmol / L sodium phosphate buffer (pH7.5), freeze and thaw repeatedly at -80°C to lyse the bacteria, add 5mmol / L L benzylamine, incubate at 30°C for 30 minutes, then add 400μL of dinitrophenylhydrazine (1mmol / L, dissolved in 3M HCl), then add 2.4mL of 1.25M NaOH, react fo...
Embodiment 3
[0081] Construction and expression of embodiment 3 monoamine oxidase recombinant bacteria
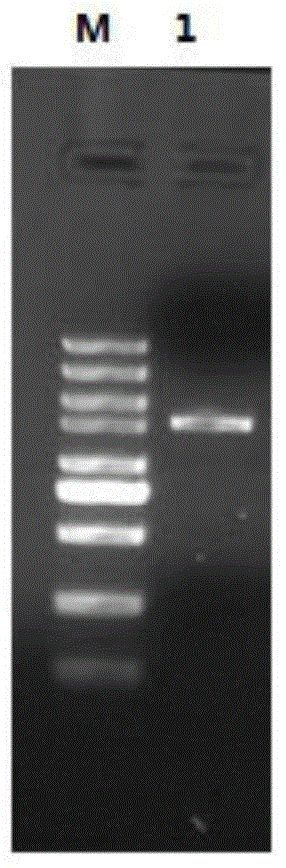
[0082] Adopt primer PF (nucleotide sequence is SEQIDNO:3) and PR (nucleotide sequence is SEQIDNO:4) PCR amplification monoamine oxidase full-length gene (results see figure 1 ), the PCR product was digested with NdeI / XhoI and connected to pET28, and transformed into E.coliBL21(DE3), cultivated on an LB plate containing kanamycin (50 μg / mL), and selected positive colonies into 100mL liquid LB medium For cultivation, the overnight culture was transferred to fresh 1 LLB culture medium and cultivated to OD 600 To 0.6 ~ 0.8, add IPTG200μM to induce protein expression, lower the temperature to 30°C and continue to culture for 24 hours. The bacteria were collected by centrifugation at 5000rpm, washed once with 0.2M pH7.0 sodium phosphate buffer, 1g of bacteria was resuspended in the above 5mL phosphate buffer, after ultrasonic disruption, the expression level was tested by SDS-PAGE.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com