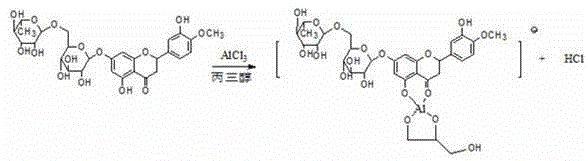
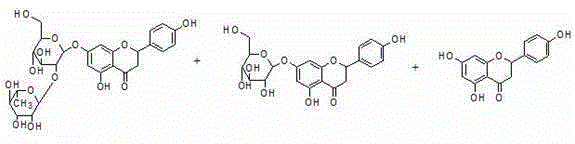
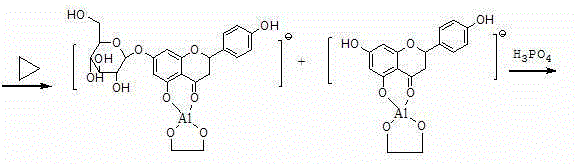
Preparation method of flavone aglycone or monoglycoside from aluminum-salt-flavonoid-glycoside complex through hydrolysis
A technology of flavone monoglycoside and flavone aglycone, which is applied in the fields of chemistry and medicine, can solve the problems of long hydrolysis time, poor selectivity, cumbersome operation, etc., and achieves simple and easy method, high yield and yield, and short hydrolysis time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Take 3.0g of anhydrous aluminum chloride, quickly add 60ml of glycerin, 10ml of ethanol, stir evenly, 20g of 92% hesperidin, stir evenly, hydrolyze under reflux at 85°C, follow-up inspection by HPLC / TLC until there is no hesperidin and Hesperetin-7-O-glucoside (about 15h, add 5ml of phosphoric acid, mix well, slowly pour the above hydrolyzate into 500ml of 1% phosphoric acid aqueous solution, statically precipitate light yellow precipitate, filter, wash, and put the filter cake in In 70% ethanol solution, heat to dissolve, add 0.1% phosphoric acid, let cool, filter with suction, wash with water, and dry at 60°C to obtain 9.1g of hesperetin, which is 93.6% determined by HPLC, melting point and UV spectrum peak shape , HPLC were consistent with the hesperetin reference substance.
[0019]
[0020]
example 2
[0022] Take 6H 2 O-aluminum trichloride 6.0g, add glycerol-ethanol (40:10) mixed solvent 50ml, 85% baicalin 10g, anhydrous calcium chloride 4.0g, heat to dissolve, add sulfuric acid 2ml, stir evenly, put Hydrolyze at 90°C under airtight reflux, follow up and check by HPLC / TLC until there is no baicalin (about 16 hours, the solution is first turbid, then clear, and then precipitate), slowly add 4ml of phosphoric acid, stir, and slowly pour the above hydrolyzate into 500ml0.5% In phosphoric acid water, precipitate precipitates, filter, wash with water, drain the filter cake, place in 50ml of 0.1% phosphoric acid methanol solution, ultrasonicate for 20min, let cool, filter, wash with cold water, and dry at 60°C to obtain baicalein 5.6 g, the content determined by HPLC is 90.4%, and the melting point, UV spectrum peak shape, and retention time of HPLC are all consistent with the baicalein reference substance.
[0023]
[0024]
example 3
[0026] Take 6H 2 Add 6.0g of O-aluminum trichloride, add 100ml of methanol solvent, take 10g of 98% rhubaricin, heat and dissolve in a closed manner at 80°C, add 6ml of hydrochloric acid, and follow up with HPLC / TLC for hydrolysis at 80°C until all rhoantisides produce apigenin (About 16 hours, the clear solution will precipitate out) Add 5ml of phosphoric acid, sonicate for 30min, filter, combine the filter cake, place in 0.2% phosphoric acid solution, sonicate for 20min, filter, wash with water, and dry at 60°C to get apigenin 4.1 g, the retention time of melting point, UV spectrum peak shape, and HPLC are all consistent with the apigenin reference substance.
[0027]
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com