Method for cooperatively removing and selectively recovering heavy metal cations and non-metal anions from wastewater
A technology of heavy metals and cations, applied in chemical instruments and methods, alkali metal compounds, alkali metal oxides/hydroxides, etc., can solve problems such as poor treatment effect, heavy secondary pollution, and poor load-bearing capacity, and achieve operational Simple, low cost, anti-leaching effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
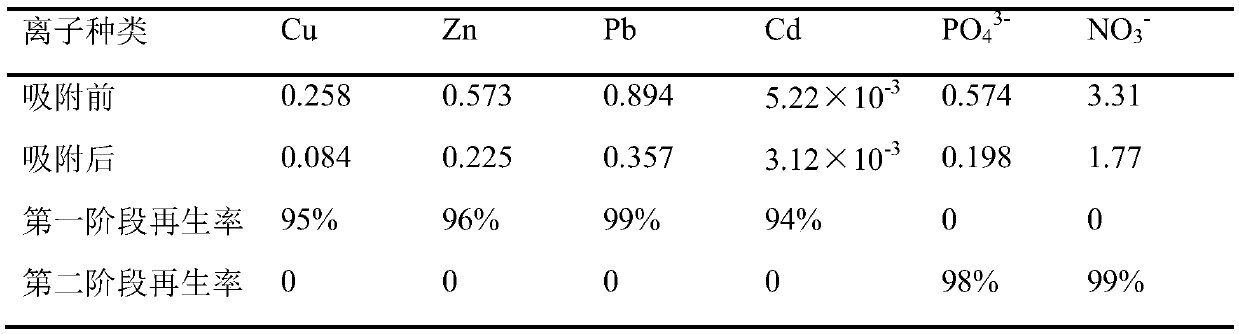
[0032] A method for synergistically removing and selectively recovering heavy metal cations and non-metal poisonous anions by using a chitosan-based composite adsorbent, the steps of which are:
[0033] (1) Synergistic adsorption: put 10mL of adsorbent into a jacketed glass adsorption column (Φ20×300mm), keep the column temperature at 25°C, and mix nickel ion and phosphate (initial concentration of nickel ion is 1mmol / L , the initial concentration of phosphate ions is 1mmol / L), adjust the pH to 5, pass through the adsorption column filled with adsorbent at a flow rate of 5BV / h, and the processing capacity is 50BV / batch. The average concentration of nickel ions in the adsorbed water is 0.721mmol / L, and the concentration of phosphate ions is 0.324mmol / L.
[0034] (2) Nickel ion recovery: raise column temperature to 30 ℃, use 20BV dilute hydrochloric acid solution (molar concentration is 0.001mmol / L, the concentration of hydrochloric acid and sodium hydroxide in the following exa...
Embodiment 2
[0041] With embodiment 1, difference is: the initial concentration of nickel ion is 1mmol / L in the step (1), and the initial concentration of phosphate ion is 2mmol / L, under the same adsorption and regeneration condition, remaining nickel ion after adsorption The concentration is 0.759mmol / L, and the phosphate ion concentration is 0.658mmol / L. The desorption rate of nickel ions in the first stage of regeneration reached 94%, and no phosphate was detected; the desorption rate of phosphate in the second stage of regeneration reached 97%.
Embodiment 3
[0043] Same as Example 1, the difference is that the column temperature in the adsorption stage in step (1) is changed to 40°C, the flow rate of the mixed solution is 30BV / h, the average concentration of nickel ions in the adsorbed water is 0.712mmol / L, and the concentration of phosphate ions is 0.303 mmol / L. The desorption effect is close to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com