Polymer containing quaternary ammonium group, anion exchange membrane and preparation method thereof
A quaternary ammonium group, polymer technology, applied in the field of quaternary ammonium group-containing polymers, anion exchange membranes and their preparation, can solve the problems of hindering ion conduction, reducing membrane toughness and mechanical processing performance, and having no ion conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a kind of preparation method of anion exchange membrane, comprises the following steps,
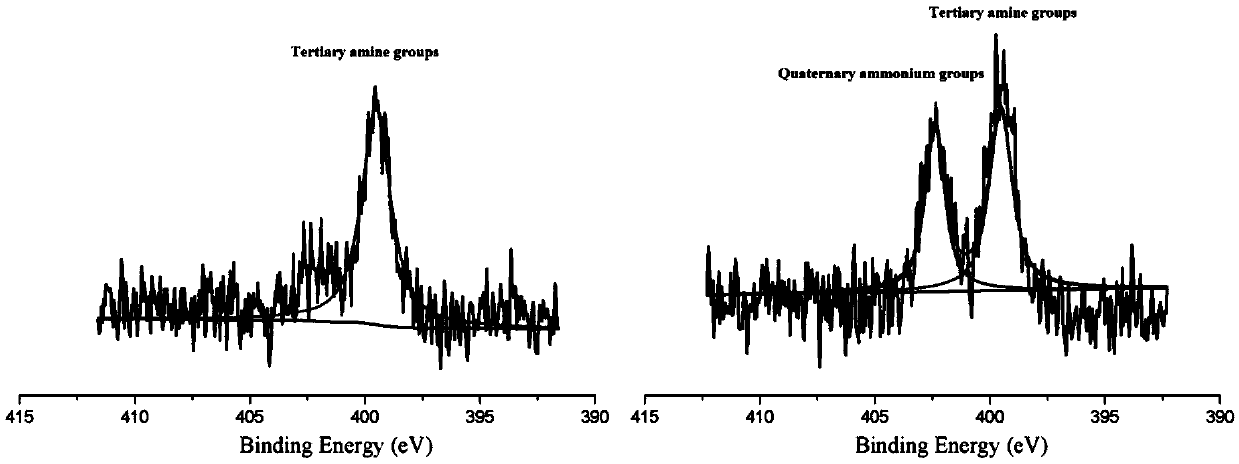
[0042] A) under a protective atmosphere, after mixing and reacting a polymer containing a tertiary amine group in the side chain, a quaternizing agent and an anhydrous polar aprotic solvent, a polymer solution containing a quaternary ammonium group is obtained;
[0043] The polymer containing tertiary amine groups in the side chain is polyarylether sulfone containing tertiary amine groups in the side chain, polyarylether ketone containing tertiary amine groups in the side chain and polyarylether sulfone ketone containing tertiary amine groups in the side chain One or more of them; the quaternizing agent is one or more of methyl iodide, (3-chloropropyl) trimethoxysilane and (3-chloropropyl) trimethoxysilane systems;
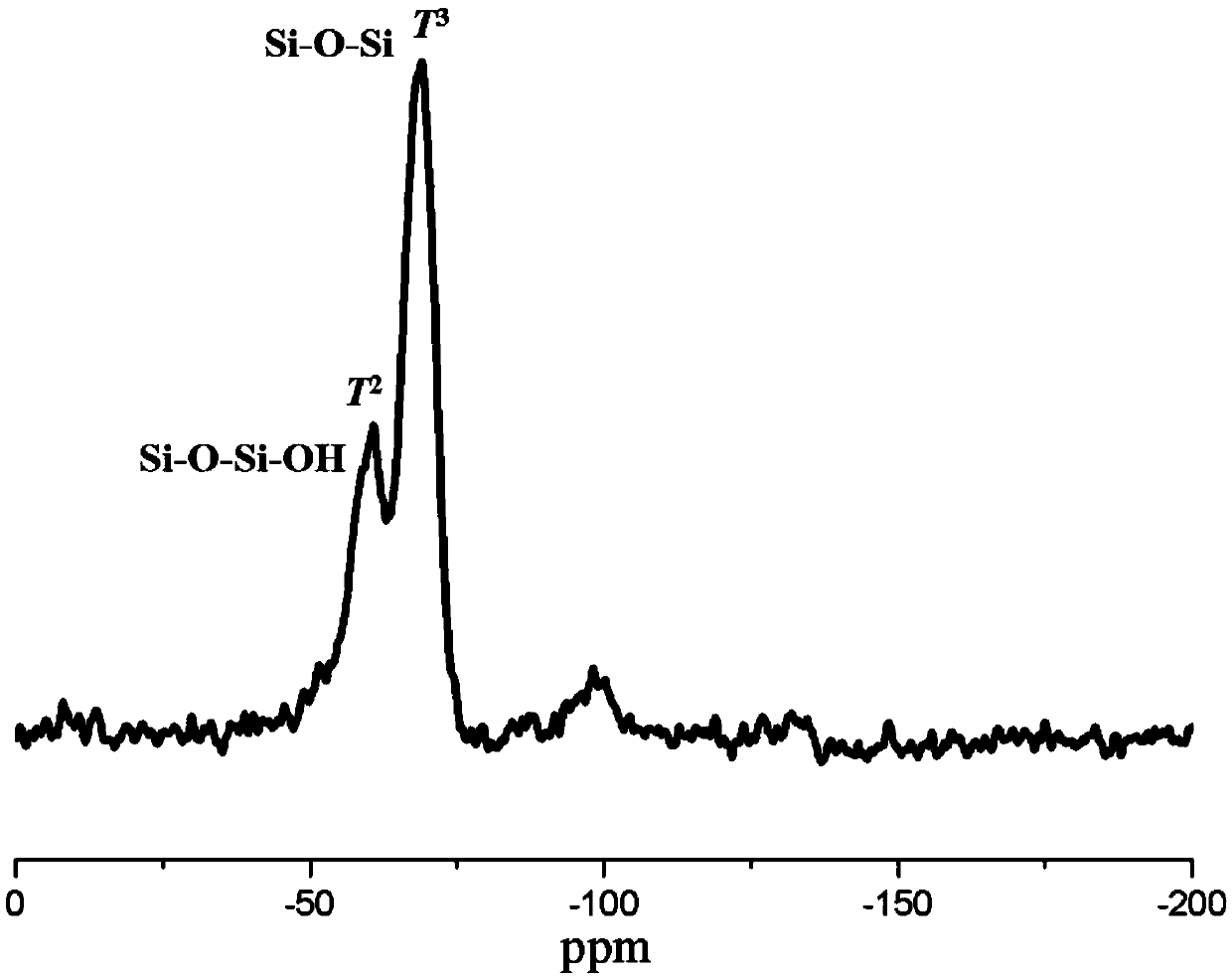
[0044] B) After adding an alcohol solvent to the polymer solution containing quaternary ammonium groups obtained in the above steps...
Embodiment 1
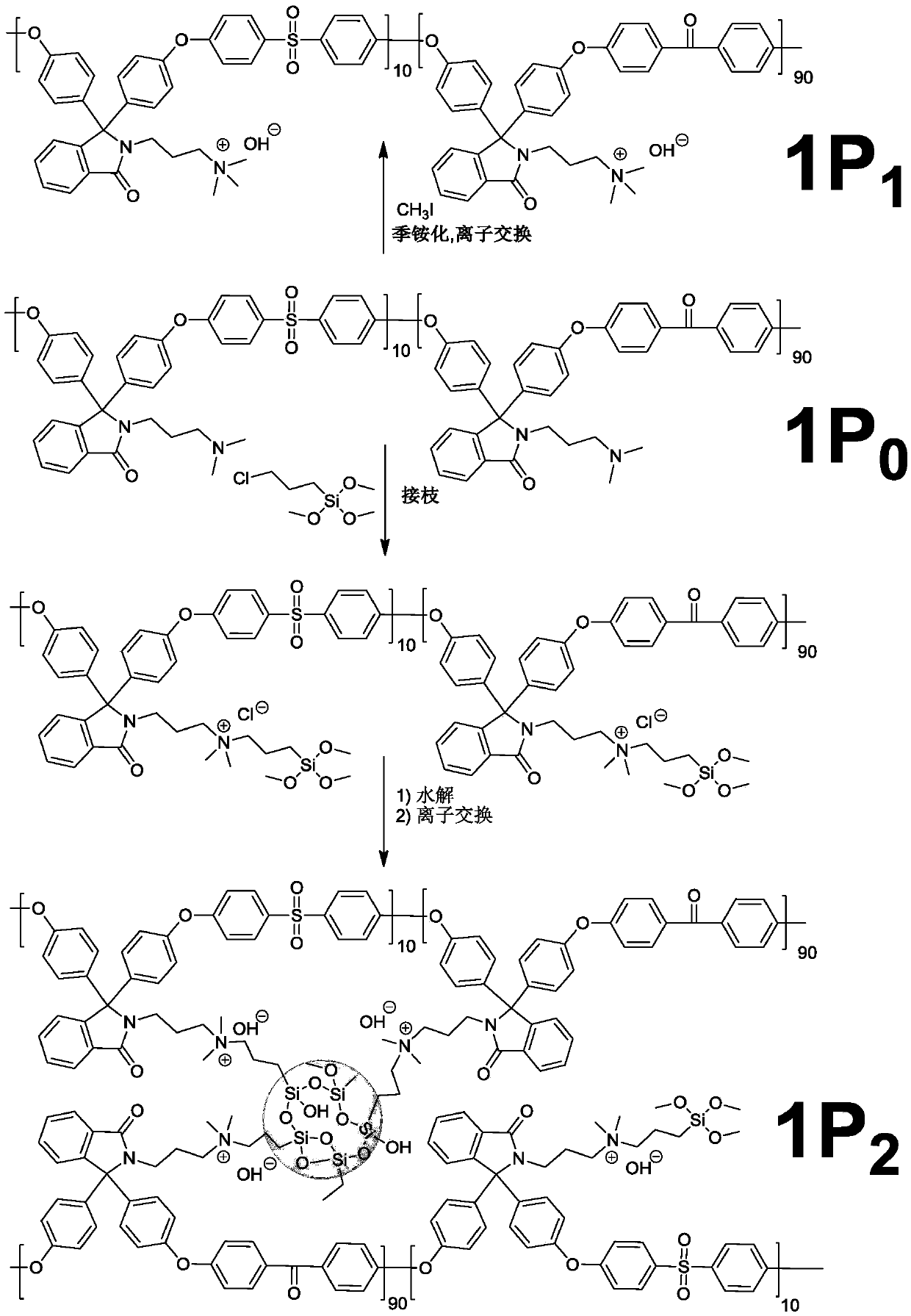
[0104] Polymer 1P 1 The preparation process
[0105] Under nitrogen protection, 7.00 grams (12.0 mmol) of tertiary amine polymer 1P 0It was dissolved in 115 mL of dry N,N-dimethylacetamide, and after stirring and dissolving completely, 1.71 g (12.0 mmol) of iodomethane was added thereto. After reacting at room temperature for 24 hours, the above-mentioned reaction solution was cast into a film in a 60°C oven. After volatilizing most of the solvent, it was heated (120°C) and vacuum-dried to remove the remaining solvent in the film, and cooled to At room temperature, a polymer film is finally obtained. The membranes were soaked in 1M sodium hydroxide solution for 48 hours for ion exchange, then soaked in deionized water for 48 hours, and washed with the solution for more than three times. Finally, the membrane was stored in deionized water until use.
Embodiment 2
[0107] 1P 2 The preparation process
[0108] Under nitrogen protection, 7.00 grams (12.0 mmol) of tertiary amine polymer P 0 Dissolve in 115mL of dry N,N-dimethylacetamide, after stirring and dissolving completely, add 2.37g (12.0mmol) of (3-chloropropyl)trimethoxysilane (CPTMS) and catalyst amount Anhydrous potassium iodide [the dosage is 0.5% (molar equivalent) of CPTMS]. The temperature was controlled at 85°C, and after 24 hours of reaction, 5 mL of 95% EtOH was added to continue the reaction (hydrolysis) for 30 minutes. The above reaction solution was cast into a film in a 60°C oven, and after most of the solvent was evaporated, it was heated (120°C) and vacuum-dried to remove the residual solvent in the film, cooled to room temperature, and finally obtained a polymer object film. The membranes were soaked in 1M sodium hydroxide solution for 48 hours for ion exchange, then soaked in deionized water for 48 hours, and washed with the solution for more than three times. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com