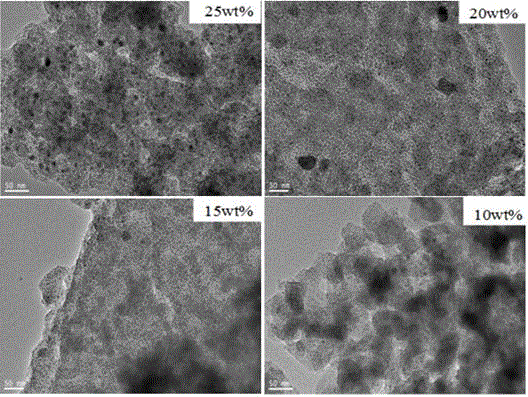
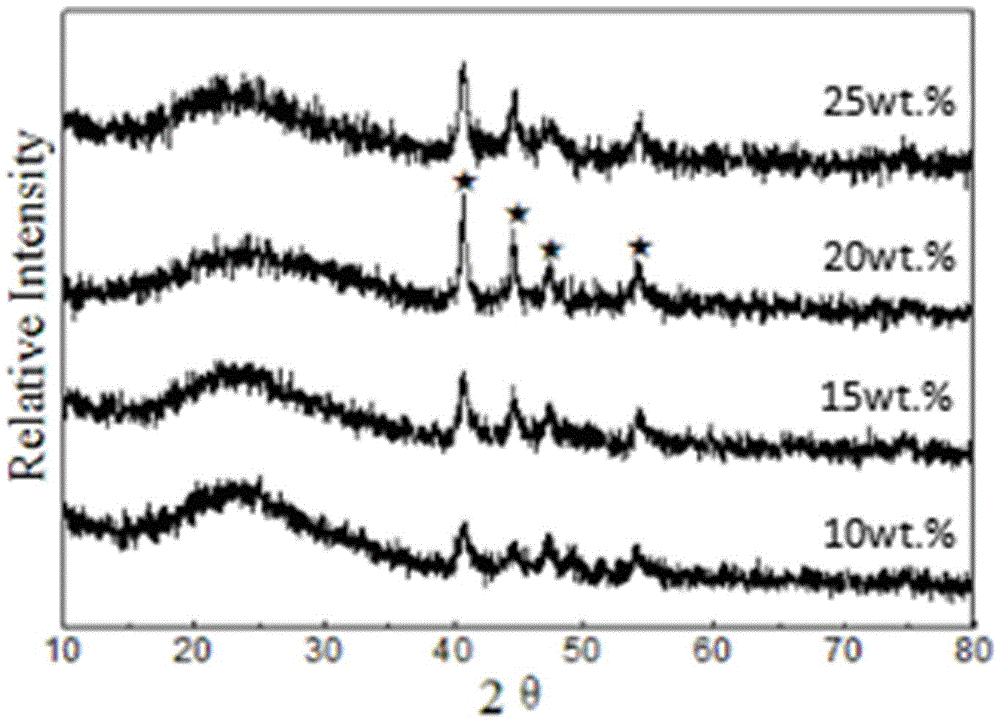
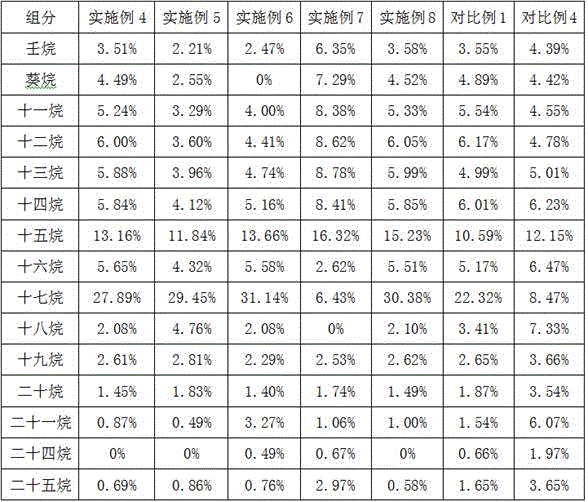
Preparing of Ni2P/Zr-MCM-41 catalyst and application for preparing biofuel by catalyzed biolipid
A zr-mcm-41, catalyst technology, applied in physical/chemical process catalysts, biological raw materials, molecular sieve catalysts and other directions, can solve the problems of affecting catalytic performance, affecting catalytic effect, small specific surface area, etc. Good resistance to carbon deposition and stability, the effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Preparation of Zr-MCM-41 carrier
[0034] Add cetyltrimethylammonium bromide (CTAB) into water, stir until completely dissolved, then add zirconium nitrate and tetraethyl orthosilicate (TEOS), and adjust the pH to 10.5 with ethylenediamine, stir for 2 hours, and then heat up to 105°C, and crystallized at this temperature for 48h. After the reaction, the sample obtained was washed with water until neutral, and dried at room temperature. Then the obtained sample was placed in a muffle furnace, heated to 550°C in an air atmosphere, and roasted for 6 hours to remove the surfactant to obtain a Zr-MCM-41 carrier; wherein cetyltrimethylammonium bromide: B The molar ratio of diamine: water: tetraethylorthosilicate: zirconium nitrate is 0.14:8.0:130:1:0.025;
[0035] 2. Ni 2 Preparation of P / Zr-MCM-41 Catalyst Precursor
[0036] Weigh nickel nitrate and diammonium hydrogen phosphate according to the molar ratio of Ni:P=1, mix them, 2 P20% load was made into an aqueous sol...
Embodiment 2
[0040] 1. Preparation of Zr-MCM-41 carrier: Same as Example 1.
[0041] 2. Ni 2 Preparation of P / Zr-MCM-41 catalyst precursor: same as Example 1.
[0042] 3. Ni 2 Preparation of P / Zr-MCM-41 Catalyst
[0043] will Ni 2 The P / Zr-MCM-41 catalyst precursor is reduced with hydrogen. The specific operation is: first rise to 250°C at 10°C / min in a hydrogen atmosphere (flow rate 100mL / min), and then rise to 350°C at 5°C / min. Then rise to 550°C at 1°C / min and keep at 550°C for 2h. After hydrogen reduction, the product was cooled to room temperature and switched to a low concentration of O 2 After passivation for 2 hours, Ni with a loading capacity of 20wt% was obtained 2 P / Zr-MCM-41 catalyst.
Embodiment 3
[0045] 1. Preparation of Zr-MCM-41 carrier: Same as Example 1.
[0046] 2. Ni 2 Preparation of P / Zr-MCM-41 catalyst precursor: same as Example 1.
[0047] 3. Ni 2 Preparation of P / Zr-MCM-41 Catalyst
[0048] will Ni 2 The P / Zr-MCM-41 catalyst precursor is reduced with hydrogen. The specific operation is: first rise to 250°C at 10°C / min in a hydrogen atmosphere (flow rate 100mL / min), and then rise to 350°C at 5°C / min. Then increase to 600°C at 1°C / min and keep at 600°C for 2h. After hydrogen reduction, the product was cooled to room temperature and switched to a low concentration of O 2 After passivation for 2 hours, Ni with a loading capacity of 20wt% was obtained 2 P / Zr-MCM-41 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com