Method for preparing alicyclic ketone by catalytic oxidation of alicyclic alcohol compound
A technology for compounds and alicyclic alcohols is applied in the field of catalytic reaction systems for selectively oxidizing alicyclic alcohol compounds to prepare corresponding alicyclic ketones, and can solve the problems of strong halogen corrosion, large amount of waste residue, pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
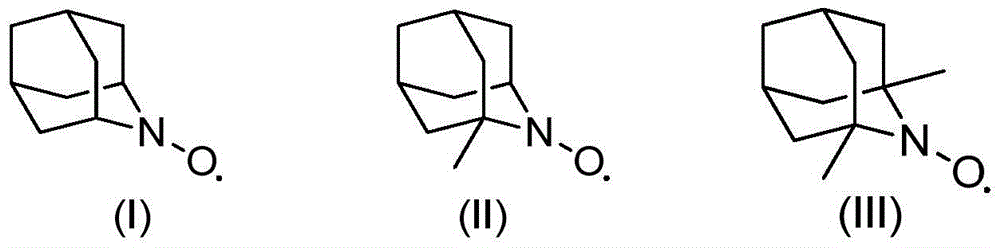
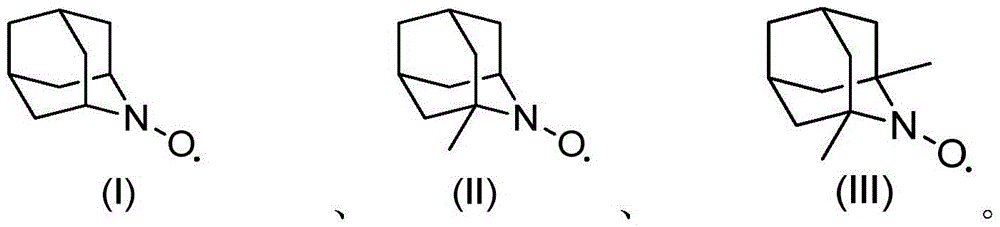
[0013] 1.0g cyclohexanol, 2mol% (relative to the substrate cyclohexanol) azaadamantane type nitroxide free radical (I), 2mol% (relative to the substrate cyclohexanol) vanadyl sulfate, 5mL acetonitrile was added to The reactor was filled with oxygen at a pressure of 0.3 MPa, operated at 80°C for 3 hours, and then cooled to room temperature. Sampling was analyzed by gas chromatography, and the conversion rate of cyclohexanol was 99.5%, and the selectivity of cyclohexanone was 99.9%.
[0014] If use 2,2,6,6-tetramethylpiperidine nitroxide free radical to replace azaadamantane type nitroxide free radical (I), under the same reaction conditions of above-mentioned embodiment 1 (catalyst substrate ratio, reaction temperature , reaction time, oxygen partial pressure, solvent and consumption etc.), the conversion rate of cyclohexanol is only 67%. If the reaction time is further extended to 15 hours, the conversion of cyclohexanol reaches 91%. It can be seen that its oxidation efficie...
Embodiment 2
[0016] With 10g cyclohexanol, 1mol% (relative to the substrate cyclohexanol) azaadamantane type nitroxide radical (I), 1mol% (relative to the substrate cyclohexanol) vanadyl sulfate, 100mL acetonitrile was added to the reaction The kettle was filled with oxygen at a pressure of 0.5MPa, operated at 100°C for 10h and then cooled to room temperature. Sampling was analyzed by gas chromatography, and the conversion rate of cyclohexanol was 99.1%, and the selectivity of cyclohexanone was 99.9%.
Embodiment 3
[0018] With 1.76g2-phenyl cyclohexanol, 0.05mol% (relative to the substrate 2-phenylcyclohexanol) azaadamantane type nitroxide free radical (II), 0.5mol% (relative to the substrate 2-benzene Base cyclohexanol) vanadyl trichloride, 5mL of acetonitrile was added to the reaction kettle, filled with air pressure of 0.5MPa, operated at 80°C for 20h and then cooled to room temperature. Samples were analyzed by gas chromatography, and the conversion rate of 2-phenylcyclohexanol was 99.7%, and the selectivity of 2-phenylcyclohexanone was 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com