Preparation method of biodegradable polyurethane acrylate carrier released by coating medicine
A polyurethane acrylate, biodegradable technology, used in coatings, prostheses, medical science and other directions, can solve problems such as low utilization rate, and achieve the effect of improving hydrophilicity and improving degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
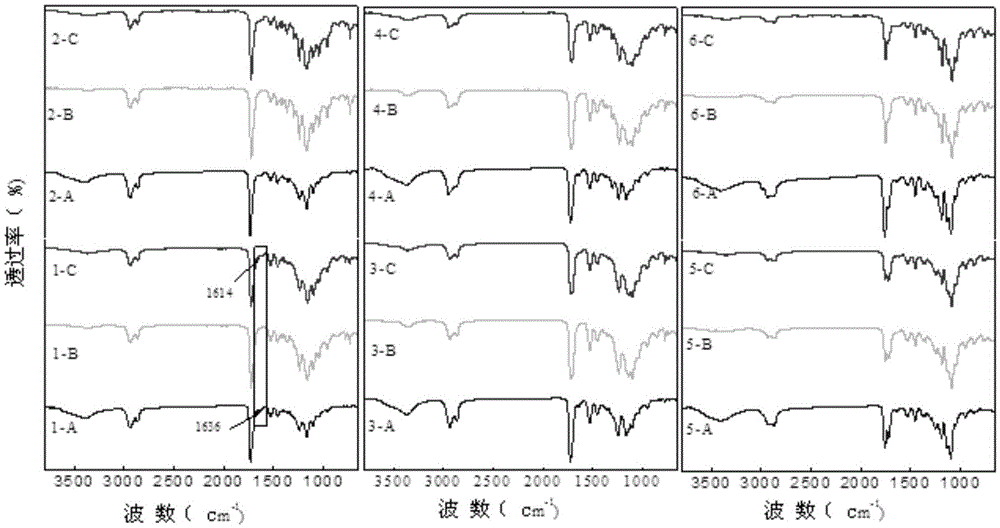
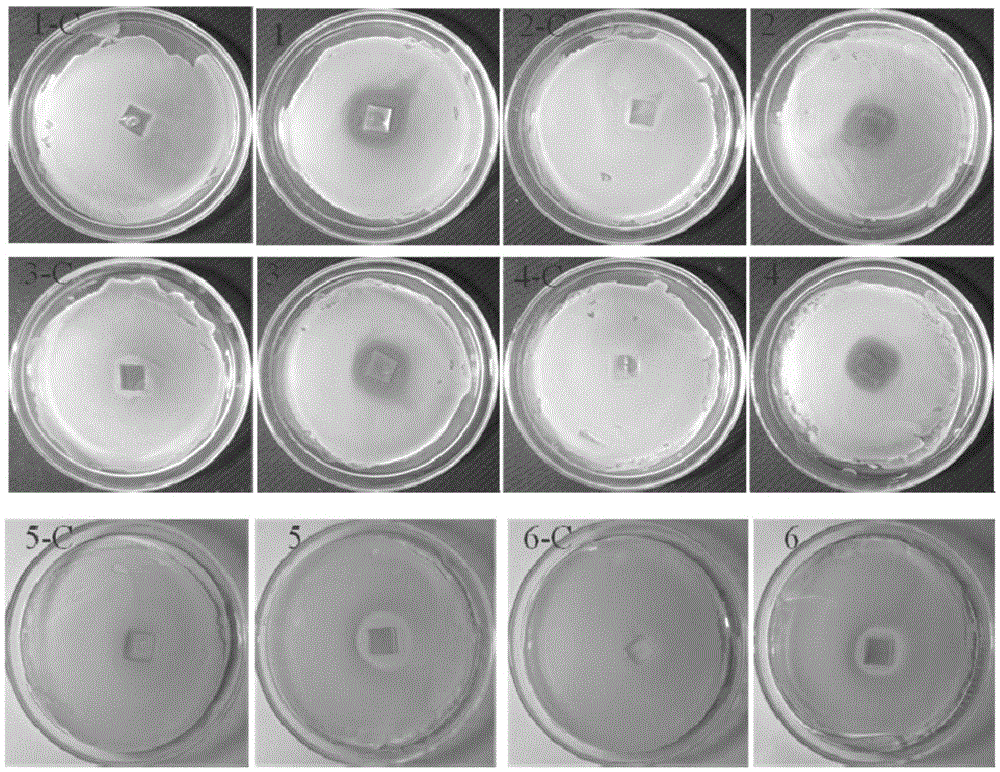
Image
Examples
Embodiment 1
[0022] (1) Synthesis of polyether ester diol: Add 5.00 g of initiator polyethylene glycol (Mw400) into a 100 mL round bottom flask, vacuumize at 100 ° C for 3 h, then cool down to room temperature, and add ε-caprolactone 32.50g, add catalyst stannous octoate 0.1125g, pass into N 2 Raise the temperature to 130°C, react for 24 hours, and stop the reaction. The obtained product was poured into excess cold n-hexane and diethyl ether, centrifuged and precipitated, dried in a vacuum oven at 50°C for 24 hours, and transferred to a drying tower for later use.
[0023] (2) Preparation of polyurethane acrylate: Add 0.005mol of polyether ester diol to a 100mL round-bottomed flask and vacuumize for 3 hours at 100°C, then cool down to room temperature, and add 0.01mol of hexamethylene diisocyanate and catalyst organic bismuth in turn 0.0500g, reacted at 80°C under nitrogen protection for 3h. The reaction system was cooled to 45°C, and 0.01mol hydroxyethyl methacrylate was added to contin...
Embodiment 2
[0026] (1) Synthesis of polyether ester diol: Add 5 g of polyethylene glycol (Mw400) starter into a 100 mL round bottom flask, vacuumize at 100 °C for 3 h, then cool down to room temperature, add ε-caprolactone 20 g , add 0.075g catalyst stannous octoate, feed N 2 Raise the temperature to 130°C, react for 24 hours, and stop the reaction. The obtained product was poured into excess cold n-hexane and diethyl ether, centrifuged and precipitated, dried in a vacuum oven at 50°C for 24 hours, and transferred to a drying tower for later use.
[0027] (2) Preparation of polyurethane acrylate: Add 0.01mol of the obtained polyether ester diol into a 100mL round-bottomed flask and vacuumize for 3 hours at 100°C, then cool down to room temperature, and add 0.02mol of hexamethylene diisocyanate and catalyst in turn Organic bismuth 0.0700g was reacted at 80°C under nitrogen protection for 3h. Cool the reaction system to 45° C., add 0.02 mol hydroxyethyl methacrylate to continue the reacti...
Embodiment 3
[0030](1) Synthesis of polyether ester diol: Add 5 g of polyethylene glycol (Mw400) starter into a 100 mL round bottom flask, vacuumize at 100 °C for 3 h, then cool down to room temperature, add ε-caprolactone 20 g , add 0.075g of catalyst stannous octoate, pass into N 2 Raise the temperature to 130°C, react for 24 hours, and stop the reaction. The obtained product was poured into excess cold n-hexane and diethyl ether, centrifuged and precipitated, dried in a vacuum oven at 50°C for 24 hours, and transferred to a drying tower for later use.
[0031] (2) Preparation of polyurethane acrylate: Add 0.01mol of the obtained polyether ester diol into a 100mL round-bottomed flask and vacuumize for 3 hours at 100°C, then cool down to room temperature, and add 0.02mol of isophorone diisocyanate and catalyst organic Bismuth 0.0733g was reacted at 80°C under nitrogen protection for 3h. Cool the reaction system to 45° C., add 0.02 mol hydroxyethyl methacrylate to continue the reaction a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com