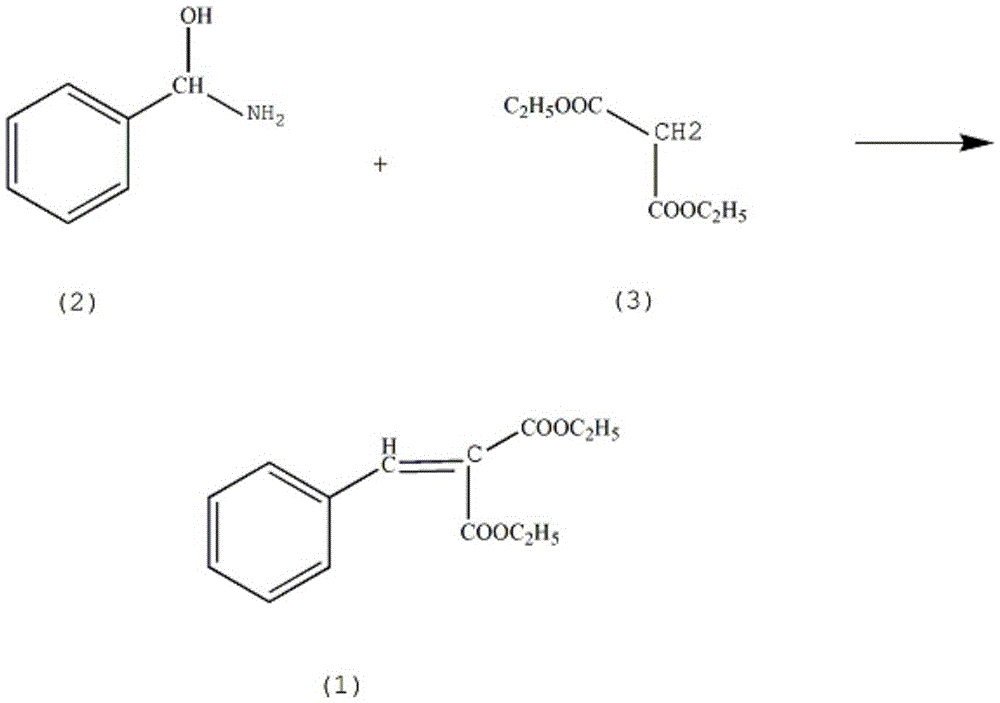
Method for synthesizing carbenicillin sodium pharmaceutical intermediate diethyl(phenylmethylene)malonate
A technology of diethyl benzylidene malonate and carbenicillin sodium, which is applied in the field of synthesis of diethyl benzylidene malonate, a pharmaceutical intermediate of carbenicillin sodium, can solve the problems of weakened antibacterial activity, etc. Achieve the effects of reducing intermediate links, reducing reaction temperature and reaction time, and increasing reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] Add 1.3mol of diethyl malonate (3) in the reaction vessel, the mass fraction is 70% benzylamine alcohol solution 1.6mol, the mass fraction is 40% cyclohexane 500ml, aluminum oxide 1.5mol, the mass fraction is 60% Toluene 200ml, raise the solution temperature to 80°C, reflux for 5h, lower the solution temperature to 60°C, continue the reaction for 80min, lower the solution temperature to 10°C, let stand for 2h, separate the solution, take out the oil layer, the mass fraction is 35% oxalic acid solution Washing, washing with potassium sulfite solution, extracting 5 times with a xylene solution with a mass fraction of 85%, combining the extracts, dehydrating phosphorus pentoxide, and distilling under reduced pressure at 0.3kPa to collect fractions at 110--117°C. Recrystallized in 91% acetonitrile to obtain 267.59 g of crystalline diethyl benzylidene malonate with a yield of 83%.
example 2
[0015] Add 1.3mol of diethyl malonate (3) in the reaction vessel, the mass fraction is 72% benzylamine alcohol solution 1.7mol, the mass fraction is 42% cyclohexane 500ml, aluminum oxide 1.5mol, the mass fraction is 62% Toluene 200ml, raise the solution temperature to 82°C, reflux for 6h, lower the solution temperature to 63°C, continue the reaction for 92min, lower the solution temperature to 12°C, let stand for 3h, separate the solution, take out the oil layer, the mass fraction is 38% oxalic acid solution Washing, washing with potassium sulfite solution, extracting 6 times with a mass fraction of 83% xylene solution, combining the extracts, dehydrating with anhydrous potassium carbonate, and distilling under reduced pressure at 0.31kPa to collect fractions at 110--117°C. Recrystallized in 93% acetonitrile to obtain 277.26 g of crystalline diethyl benzylidene malonate with a yield of 86%.
example 3
[0017] Add 1.3mol of diethyl malonate (3) in the reaction vessel, the mass fraction is 75% benzylamine alcohol solution 1.9mol, the mass fraction is 45% cyclohexane 500ml, aluminum oxide 1.5mol, the mass fraction is 65% Toluene 200ml, raise the solution temperature to 85°C, reflux for 8h, lower the solution temperature to 65°C, continue the reaction for 110min, lower the solution temperature to 17°C, let stand for 5h, separate the solution, take out the oil layer, the mass fraction is 40% oxalic acid solution Washing, washing with potassium sulfite solution, extracting 7 times with a mass fraction of 90% xylene solution, combining the extracts, dehydrating phosphorus pentoxide, and distilling under reduced pressure at 0.32kPa to collect fractions at 110--117°C. Recrystallized in 97% acetonitrile to obtain 293.38 g of crystalline diethyl benzylidene malonate with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com