Lysosome targeted fluorescent probe and preparation method and application thereof
A technology of lysosomes and compounds, applied in biochemical equipment and methods, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problems of large background signal, poor selectivity, low sensitivity, etc., achieve high anti-interference ability, good Effects of reversibility, fluorescence signal stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
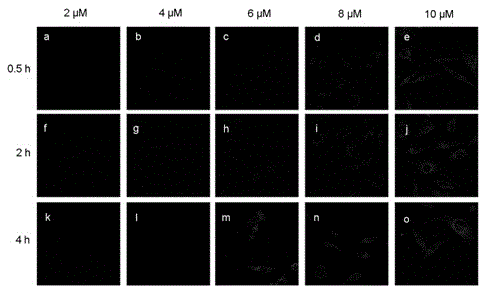
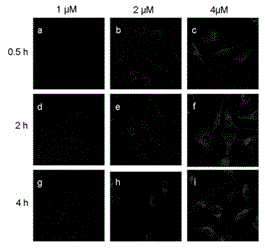
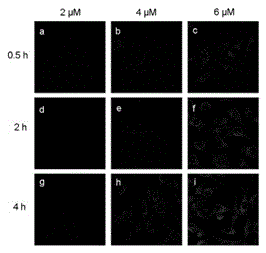
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis of probe RS1
[0044]
[0045](1) Synthesis of rhodamine B acid chloride: In a 50ml dry round bottom flask, add 0.48g (1.0mmol) rhodamine B, 5ml dry 1,2-dichloroethane and 1.0ml phosphorus oxychloride, install Condensation drying device and tail gas treatment device, control the reaction temperature at 90°C for 8 hours. After the reaction, the reaction mixture was cooled to room temperature, and the solvent was removed to obtain a dark red viscous solid for future use.
[0046] (2) Synthesis of RS1: Take 2.5ml of dry acetonitrile, dissolve all the solids obtained in the above (1), and dissolve 1.0ml of triethylamine, 1.2mmol (about 0.13g) of 2,6-di A mixture of aminopyridine in acetonitrile (5ml) was slowly added dropwise to the above reaction solution, and the reaction mixture was refluxed at 82°C for 6 hours. After the reaction, the reaction solution was cooled to room temperature, the solvent was removed, and the product was extracted...
Embodiment 2
[0051] Embodiment 2: the synthesis of probe RS2
[0052]
[0053] (1) Preparation of Compound 2: Dissolve 0.5ml (4.85mmol) acetylacetone and 0.5g (4.85mmol) 2,6-diaminopyridine in 2.5ml phosphoric acid, and reflux for 2 hours under magnetic stirring. After the reaction is complete, cool the reaction mixture to room temperature, pour it into 100ml of ice-water mixture, adjust the pH to neutral with concentrated ammonia water, obtain a large amount of precipitate, filter it with suction, wash it with water several times, and dry it to obtain an off-white crude product. The target product was obtained by column chromatography with a volume ratio of 20:1.
[0054] Compound 2: off-white solid Yield: 70.3%.
[0055] 1 HNMR(400MHz,MeOD):δ8.06(dd,J=8.9,2.8Hz,1H),6.95(s,1H),6.80(d,J=8.9Hz,1H),4.91(s,2H),2.54 (d,J=6.3Hz,6H).
[0056] 13 CNMR (101MHz, MeOD) δ160.76, 160.50, 155.73, 146.21, 134.07, 119.12, 114.58, 111.54, 23.17, 16.50.
[0057] MS (ESI) calad.forC 10 h 11 N 3 ...
Embodiment 3
[0064] Embodiment 3: the synthesis of probe RS3
[0065]
[0066] Dissolve 0.53g RS1 (1.0mmol) and 0.15g (1.2mmol) salicylaldehyde in 10ml absolute ethanol, stir and reflux for 8 hours. After the reaction was completed, the reaction mixture was cooled to room temperature and spin-dried, and the crude product was purified by column chromatography with dichloromethane and methanol at a volume ratio of 30:1.
[0067] Probe RS3: Yellow solid 0.32 g, yield 50.3%.
[0068] 1 HNMR (400MHz, CDCl 3 )δ13.57(s,1H),8.86(s,1H),8.50(d,J=8.3Hz,1H),8.00(d,J=6.8Hz,1H),7.73–7.63(m,2H), 7.42(s,2H),7.40–7.35(m,1H),7.10(d,J=7.2Hz,1H),7.04–6.94(m,2H),6.86(d,J=7.5Hz,1H),6.54 (d,J=8.8Hz,2H),6.38(d,J=2.5Hz,2H),6.16(dd,J=8.8,2.5Hz,2H),3.29–3.17(m,8H),1.04(t, J=7.0Hz,12H).
[0069] 13 CNMR (101MHz, CDCl 3 )δ169.03,165.16,161.92,154.96,154.45,152.18,150.03,148.35,139.31,133.82,133.32,133.22,128.82,128.04,127.40,124.06,123.38,119.51,118.80,117.14,115.83,115.18,108.46,107.70,97.91 ,66.10,44.25,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com