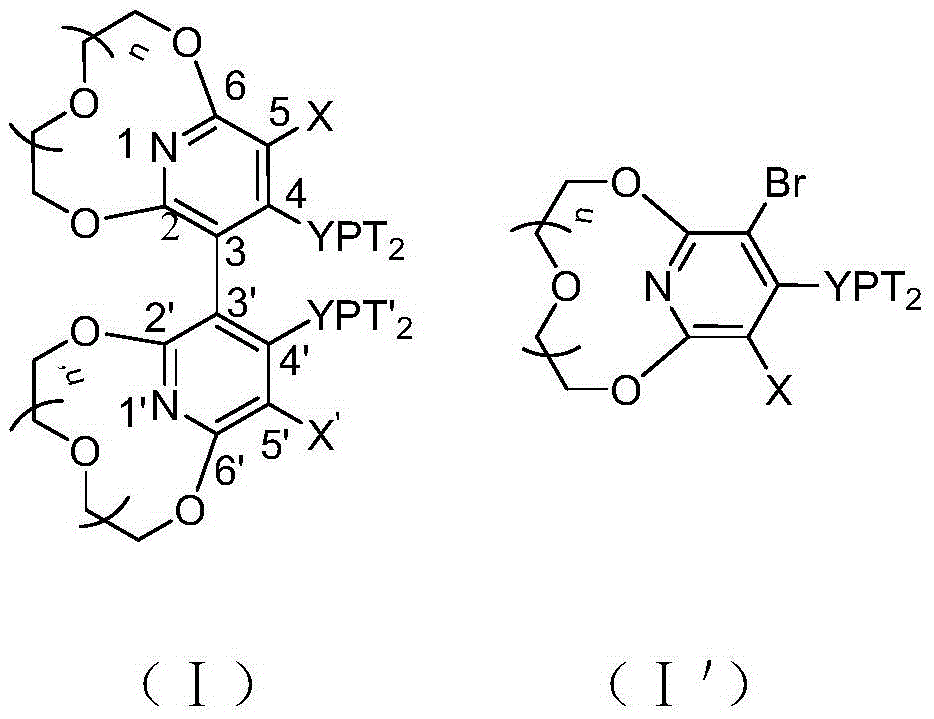
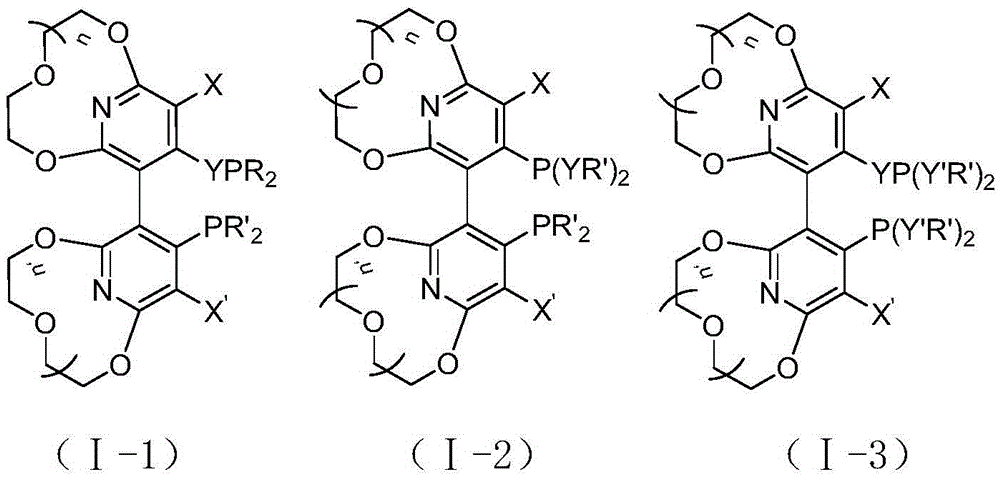
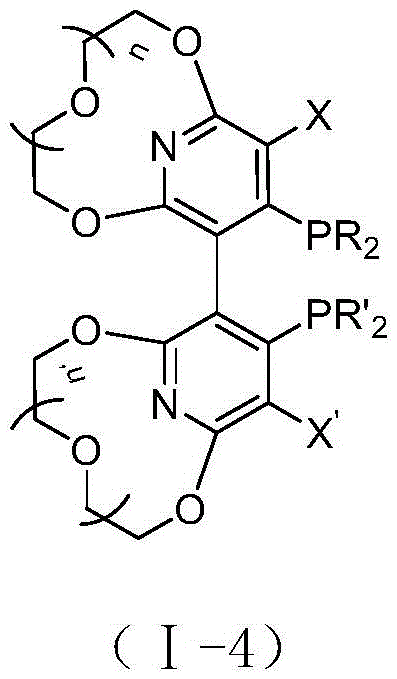
Novel pyridyl crown ether-containing chiral diphosphine ligand and application thereof in asymmetric catalytic reaction
A technology of pyridyl crown ether and pyridyl bisphosphorus, which is applied in the field of novel chiral bisphosphorus ligands containing pyridyl crown ether and its application in asymmetric catalytic reactions, and can solve the problems of inability to adjust the ligand structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Preparation of pyridyl crown ether compound II by 2,6-dichloropyridine
[0078]
[0079] Add NaH (1.92g, 80.0mmol) and KPF to a dry 500mL three-necked flask 6 (3.86g, 21mmol), N 2 Add THF (200mL) under protection, heat to reflux, slowly drop a THF solution (100mL) of 2,6-dichloropyridine (2.96g, 20.0mmol) and tetraethylene glycol (3.88g, 20.0mmol) into the reaction solution . After the addition was completed, the reaction was continued under reflux for three days. After cooling to room temperature, the excess NaH was quenched with methanol and water, the solvent was evaporated under reduced pressure, and the 2 Cl 2 (100mL) and water (70mL) to dissolve the reactant, extract and separate, and use CH 2 Cl 2 (20 mL×3) extraction. The combined organic phases were dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by column chromatography (SiO 2 :acetone / CH 2 Cl 2 =1:10) to obtain pyridyl crown ether compoun...
Embodiment 2
[0080] Embodiment 2: Preparation of monobrominated pyridyl crown ether compound III by pyridyl crown ether compound II
[0081]
[0082] Add compound II (2.45 g, 9.12 mmol) and CH in a 100 mL single-necked round bottom flask 2 Cl 2 (50mL), after the reaction solution was cooled to -70°C, CH 2 Cl 2 The solution (50 mL) was slowly added dropwise into the single-necked flask. Monitor the reaction by TLC until the raw material disappears, and add saturated Na 2 CO 3 solution to quench the reaction with CH 2 Cl 2 The aqueous phase was extracted (30 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate, filtered, and concentrated. The crude product was purified by column chromatography (SiO 2 :acetone / CH 2 Cl 2 =1:100) to obtain the dibrominated by-product and the target product monobromopyridyl crown ether compound III (white solid, 2.71g, 85% yield). 1 HNMR (500MHz, CDCl 3 ): δ7.62(d, J=8.0Hz, 1H), 6.24(d, J=8.0Hz, 1H), 4.68(t, J=5.7Hz,...
Embodiment 3
[0083] Example 3: Preparation of monophosphine ligand IV containing pyridyl crown ether from monobromopyridyl crown ether compound III
[0084]
[0085] Add i-Pr to a 500 mL dry two-neck round bottom flask 2 NH (30.1mL, 214mmol) and THF (100mL), the reaction flask was placed in a low-temperature cooling liquid at -78°C, and the n-BuLi (84.5mL, 186mmol, 2.2Minhexane) solution was slowly added dropwise to the round bottom flask. Maintain the reaction mixture at -78°C and stir for 10 minutes, warm up to -10°C and stir for 30 minutes, then put it into a low-temperature cooling liquid at -78°C, and add monobromopyridyl crown ether compound III (49.6g, 142mmol) in THF The solution (100 mL) was slowly added dropwise to the LDA solution just prepared. After the addition was complete, the reaction mixture was stirred for 30 minutes, and then (3,5-Me 2 C 6 h 3 ) 2 PCl (43.5 g, 157 mmol) was dissolved in THF (30 mL). After the dropwise addition, the reaction mixture was slowly wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com