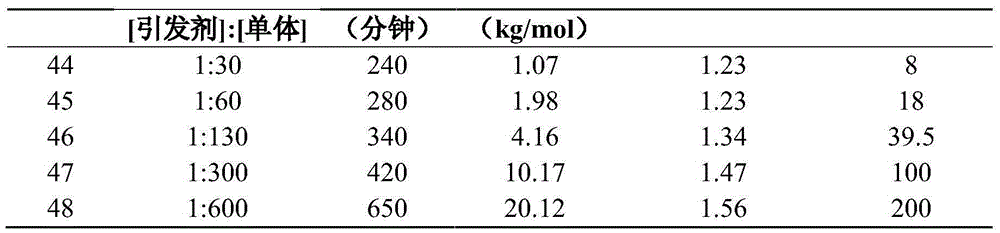
Method for preparing crosslinked polystyrene or crosslinked copolymer
A technology of cross-linked polystyrene and cross-linked copolymers, which is applied in the field of cross-linked polymer preparation to achieve the effects of simple synthesis conditions, convenient synthesis, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 polyamine ligand TPMA
[0041] Dissolve 16.4 g of 2-chloromethylpyridine hydrochloride (0.1 mol) in 40 mL of deionized water, cool in an ice bath, and slowly add 20 mL of aqueous solution in which 0.1 mol of sodium hydroxide is dissolved, and the solution turns pink. 80 mL of a DCM solution containing 5.4 g of 2-(aminomethyl)pyridine (0.05 mol) was added and warmed to room temperature. Add 20 mL of aqueous solution dissolved with 0.1 mol of sodium hydroxide with a micro-injector, and drop it in 50 hours. Stop the reaction, wash the organic phase with 3×10 mL of 15% NaOH aqueous solution, combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate. The product was extracted with diethyl ether in a boiling state, the insoluble matter was removed, cooled, the product was crystallized in diethyl ether, and filtered. The recrystallization was continued for 3 times to obtain pale yellow needle crystals with a yield...
Embodiment 2
[0042] The synthesis of embodiment 2 polyamine ligand TPEN
[0043] Dissolve 13.12 g of 2-chloromethylpyridine hydrochloride (0.08 mol) in 30 mL of deionized water, cool in an ice bath, and slowly add 15 mL of aqueous solution in which 0.08 mol of sodium hydroxide is dissolved, and the solution turns pink. Add 60 mL of a DCM solution containing 1.2 g of ethylenediamine (0.02 mol) and warm to room temperature. Add 15 mL of aqueous solution dissolved with 0.08 mol of sodium hydroxide with a micro-injector, and drop it in 50 hours. Stop the reaction, wash the organic phase with 3×10 mL of 15% NaOH aqueous solution, combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate. The product was extracted with diethyl ether in a boiling state, the insoluble matter was removed, cooled, the product was crystallized in diethyl ether, and filtered. The recrystallization was continued for 3 times to obtain pale yellow needle crystals with a yield of 37%. 1 ...
Embodiment 3
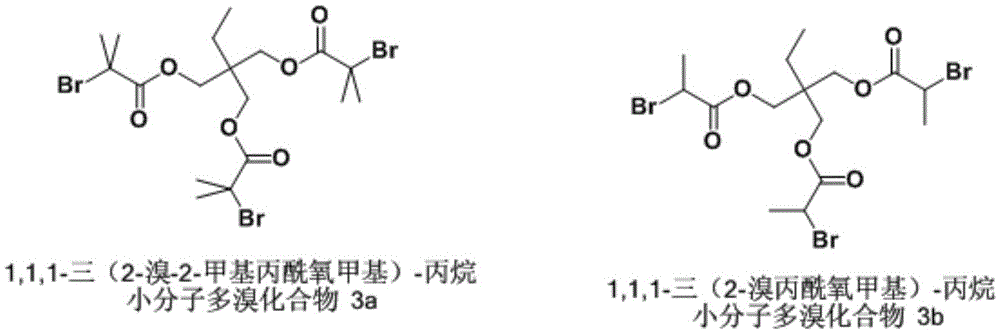
[0044] Synthesis of embodiment 3 small molecule polybrominated compound 3a
[0045] 2.7g 1,1,1-trimethylol-propane (2×10 -2 mol), 8mL triethylamine (6×10 -2 mol), 50mL of DCM were mixed, placed in a 250mL three-necked flask, and cooled in an ice bath. Will contain 8.2mL2-bromo-2-methylpropionyl bromide (6.6×10 -2 mol) in DCM (50 mL) was dropped into a three-necked flask, and after 1 hour, a white precipitate appeared, and the temperature was raised to room temperature (25° C.), and stirred overnight for 18 hours. Filter, wash the filtrate three times with 50mL1mol / LHCl solution, saturated NaHCO 3 Wash three times with aqueous solution, three times with 50mL deionized water, once with 50mL saturated NaCl, anhydrous MgSO 4 Let dry overnight. After filtration and concentration, a yellow viscous liquid was obtained. The crude product was recrystallized twice from methanol, filtered and dried under vacuum at 40°C overnight to obtain white crystals. 1 HNMR (400MHz, CDCl 3 )δ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com