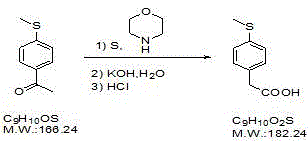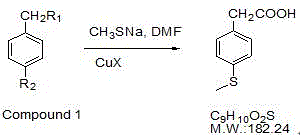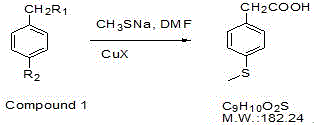Preparation method of 4-methylthio phenylacetic acid
A technology of methylthiophenylacetic acid and methylthiobenzene is applied in the field of synthesizing 4-methylthiophenylacetic acid, which can solve the problems of large environmental pollution and achieve the effects of small environmental pollution, wide sources, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 4-bromophenylacetic acid as the starting material to prepare the target compound
[0017] Take 10g of 4-bromophenylacetic acid in a 100mL three-necked flask, add 20mL of DMF, add 5.0g of sodium methyl mercaptide, add 0.1g of cuprous bromide, after nitrogen replacement, raise the reaction temperature to 130°C under stirring, and stir under nitrogen protection Reacted for 4 hours, cooled the reaction liquid, added 40% NaOH 5mL, and stirred for 10 minutes. Cool the reaction solution, add 25 mL of ethyl acetate to extract twice, add 50 mL of ethyl acetate to the water layer, add 10% dilute sulfuric acid to adjust the pH to 2-4, collect the ethyl acetate, wash the ethyl acetate with 10 mL of water, and distill out the acetic acid Ethyl ester to about 20mL, add 20mL of n-hexane, increase the reaction temperature to reflux, after all the solids are dissolved, slowly cool to room temperature, filter to obtain light yellow scaly crystals, and obtain 6.38g of the target product a...
Embodiment 2
[0019] Synthesis of target compound from ethyl 4-bromophenylacetate
[0020] Take 10g of ethyl 4-bromophenylacetate in a 100mL three-necked flask, add 20mL of DMF, add 5.0g of sodium methyl mercaptide, add 0.1g of cuprous bromide, and replace with nitrogen, raise the reaction temperature to 130°C under stirring, and protect with nitrogen Down to the end of the reaction, cool the reaction solution, add 20mL of 40% sodium hydroxide to reflux for 2 hours, cool the reaction solution, add 25mL of ethyl acetate to extract twice, add 50mL of ethyl acetate to the water layer, add 10% dilute sulfuric acid to adjust the pH to 2-4. Collect ethyl acetate, wash ethyl acetate with 10mL of water, distill off ethyl acetate to about 20mL, add 20mL of n-hexane, raise the reaction temperature to reflux, after all solids are dissolved, slowly cool to room temperature, filter, Light yellow scaly crystals were obtained, and after drying, 5.17g of the target product was obtained, yield: 65.6%
Embodiment 3
[0022] Synthesis of target compound from 4-bromophenylacetonitrile
[0023] Take 10g of 4-bromophenylacetonitrile in a 100mL three-necked flask, add 20mL of DMF, add 5.0g of sodium methyl mercaptide, add 0.1g of cuprous bromide, and replace with nitrogen, raise the reaction temperature to 130°C under stirring, and under the protection of nitrogen to After the reaction, cool the reaction liquid to room temperature, add 20 mL of 50% sulfuric acid, reflux for 5 h, cool the reaction liquid, add 25 mL of ethyl acetate to extract three times, combine the ethyl acetate layer, add 100 mL of 5% sodium hydroxide to the ethyl acetate layer, and stir After 5 minutes, the aqueous layer was collected. Add 100 mL of ethyl acetate to the water layer, add 10% dilute sulfuric acid to adjust the pH to 2-4, separate the ethyl acetate layer, wash with 10 mL of water, distill off the ethyl acetate to about 20 mL, add 20 mL of n-hexane, and increase the reaction temperature To reflux, after all the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com