Amphiphilic binary molecular brush polymer and acid-sensitive targeting nanocapsule prepared from same
A technology of binary molecules and brushing polymers, which is applied in the direction of capsule delivery, microcapsules, nanocapsules, etc., can solve the problems of difficult release of substances inside capsules, difficult application of capsules, and structural changes, etc., to achieve large coating volume, The effect of reducing toxic side effects and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
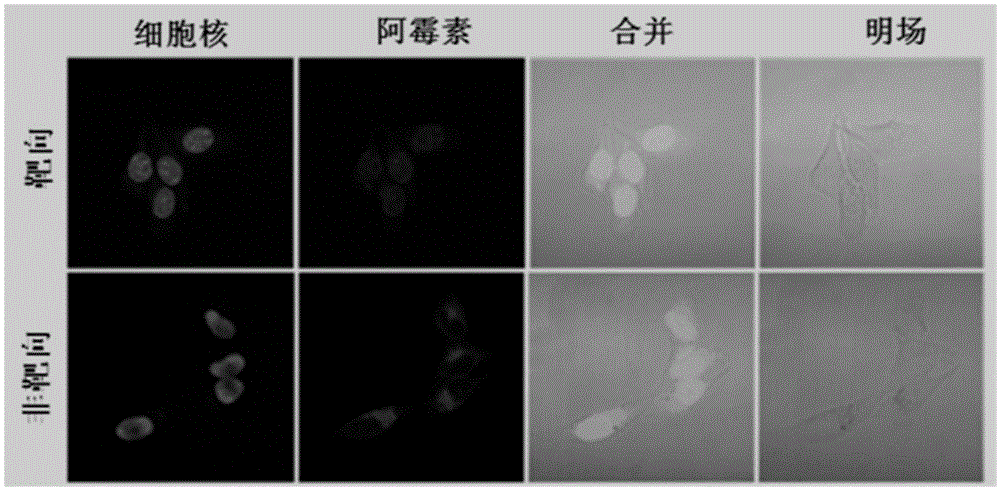
Image
Examples
Embodiment 1
[0040] An amphiphilic binary molecular brush polymer is prepared by the following steps:
[0041] (1)P(HEMA-N 3 ) main chain synthesis
[0042] According to the ratio of substances, take 1 part of 2-bromoisobutyrate monomethoxyethyl initiator, 100 parts of hydroxyethyl methacrylate (HEMA), 100 parts of methanol, 1 part of CuCl and 1 part of 2,2' - bipyridine, carry out ATRP reaction at 50° C. under the protection of nitrogen to obtain PHEMA with a degree of polymerization (DP) of 101.
[0043] According to the ratio of substances, 1 part of PHEMA, 150 parts of dibromoisobutyryl bromide, and 1000 parts of anhydrous pyridine were reacted overnight, and the product was concentrated and directly precipitated in water to obtain P(HEMA-Br).
[0044] According to the ratio of substances, take one part of P(HEMA-Br), three parts of NaN 3 and 100 parts of DMF, reacted at 50°C for 48h, concentrated and precipitated in water to obtain the main chain polymer P(HEMA-N 3 ).
[0045] (2...
Embodiment 2
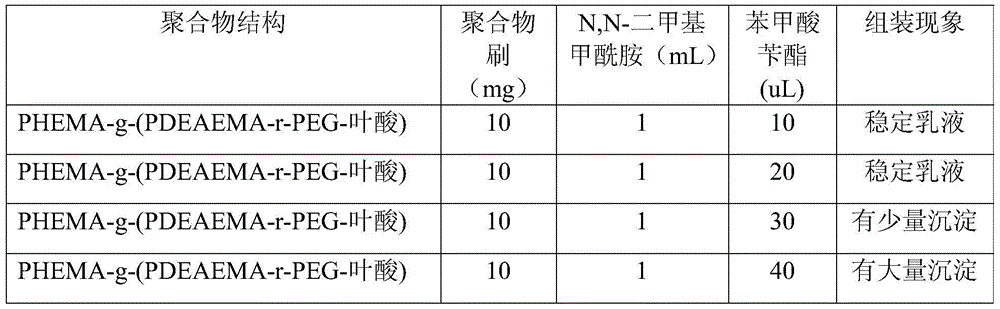
[0054] The raw materials and preparation method are the same as in Example 1, and the amount of benzyl benzoate is adjusted to explore the impact of the amount of benzyl benzoate on the stability of the capsules. The results are shown in Table 1.
[0055] The impact of the consumption of table 1 benzyl benzoate on capsule stability
[0056]
[0057] As can be seen from Table 1, when benzyl benzoate is greater than a certain value, a stable emulsion cannot be obtained.
Embodiment 3
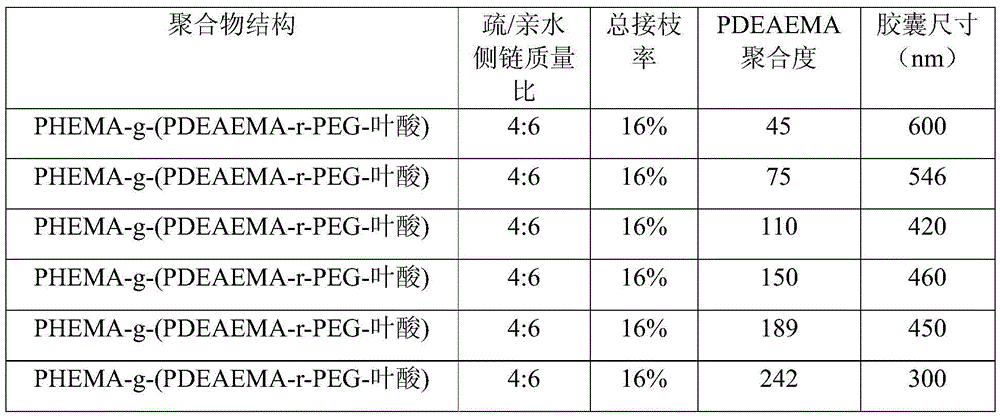
[0059] The raw materials and preparation method are the same as in Example 1, the degree of polymerization of PDEAEMA is adjusted, and the influence of the degree of polymerization of PDEAEMA on the capsule size is explored. The results are shown in Table 2.
[0060] The influence of the degree of polymerization of table 2PDEAEMA on capsule size
[0061]
[0062] It can be seen from Table 2 that responsive nanocapsules of different sizes can be prepared by adjusting the degree of polymerization of PDEAEMA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com