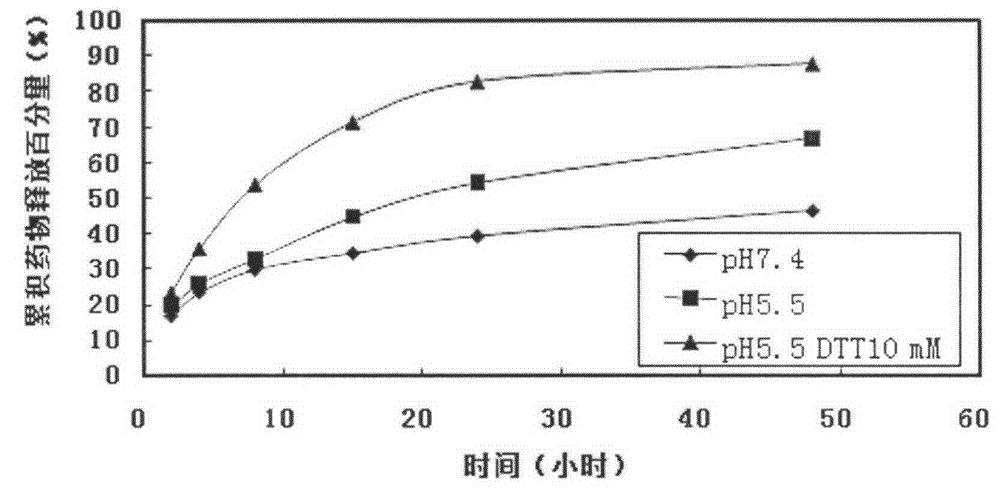
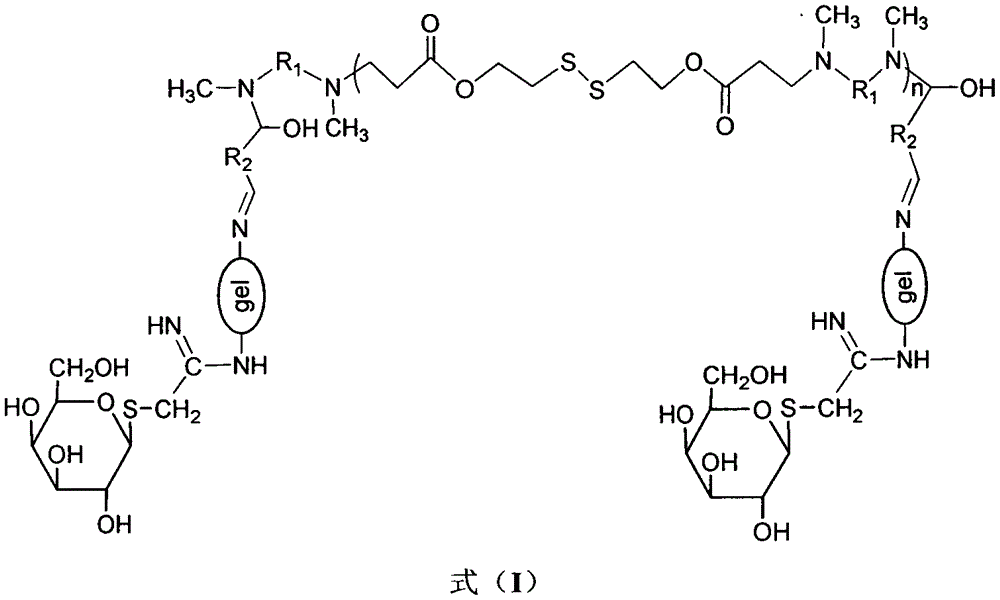
Liver-targeting drug-loaded microspheres with pH and reduction responsiveness, and preparation method and application thereof
A drug-loaded microsphere, stimuli-responsive technology, applied in the fields of polymer materials and biomedical engineering, can solve problems such as no active targeting function and inability to achieve targeted delivery of anti-tumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Preparation of galactose modified gelatin (II)
[0055] Preparation of 1-bromo-2,3,4,6-tetraacetoxy-β-D-galactose (IIb): Add 0.08ml HClO to 16ml acetic anhydride with stirring at 0°C 4 , and then slowly add 4 g of β-D-galactose (IIa) within 30 minutes, and raise the temperature to 35-40° C. for 30 minutes. Add 1.2g of red phosphorus and 2.3ml of Br successively under ice cooling 2 With 1.5ml of water, raise the temperature to 18-20°C and react for 2h. Add 20ml of chloroform, stir and pour into ice water. Filtration and liquid separation, the aqueous phase was extracted twice with 20ml chloroform, the chloroform was combined, and saturated NaHCO 3 , Wash with distilled water until the water phase is neutral. Add anhydrous MgSO 4 After drying overnight, the chloroform was removed by rotary evaporation under reduced pressure to obtain a yellow syrup sample. Dissolve it with ether and add petroleum ether until the white precipitate is just no longer dissolved, and...
Embodiment 2
[0073] Galactose-modified gelatin was prepared according to the method of Example 1.
[0074] According to the method of Example 1, replace N, N'-dimethylethylenediamine with 69.6mg N, N'-dimethylbutylene diamine, prepare the linear poly( β-amino esters).
[0075] Preparation of empty microspheres:
[0076] Dissolve 45 mg of galactose-modified gelatin and poly-β-amino ester at a mass ratio of 2:1 in 1 ml of distilled water. with saturated Na 2 CO 3 The pH of the solution was adjusted to 9 to obtain an aqueous phase.
[0077] Add 0.25ml of span80 into 50ml of liquid paraffin, stir at 85°C and 550rpm for 30min to obtain an oil phase.
[0078] At a stirring speed of 550 rpm, the water phase was added dropwise to the oil phase. After continuing to stir for 20 minutes, cool to -5°C, add 0.1g of 25% glutaraldehyde, and react at -5°C in the dark for 2h. Then add 15ml of ethanol, continue to stir for 30 minutes, filter, and wash the solid matter with ethanol and petroleum ether f...
Embodiment 3
[0084] Galactose-modified gelatin was prepared according to the method of Example 1.
[0085] According to the method of Example 1, 86.4 mg N, N'-dimethylhexamethylenediamine is used to replace N, N'-dimethylethylenediamine to prepare a linear poly( β-amino esters).
[0086] Preparation of empty microspheres:
[0087] Dissolve 50 mg of galactose-modified gelatin and poly-β-amino ester at a mass ratio of 1:2 in 2 ml of distilled water. with saturated Na 2 CO 3 The pH of the solution was adjusted to 9 to obtain an aqueous phase.
[0088] Add 0.5ml of span80 into 10ml of liquid paraffin, stir at 25°C and 50rpm for 30min to obtain an oil phase.
[0089] At a stirring speed of 50 rpm, the water phase was slowly added dropwise to the oil phase. After continuing to stir for 20 minutes, cool to 5°C, add 62.5 mg of 40% glyoxal, and react at 5°C in the dark for 2 hours. Then add 15ml of methanol, continue to stir for 30min, filter, and wash the solid matter with methanol and petr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com