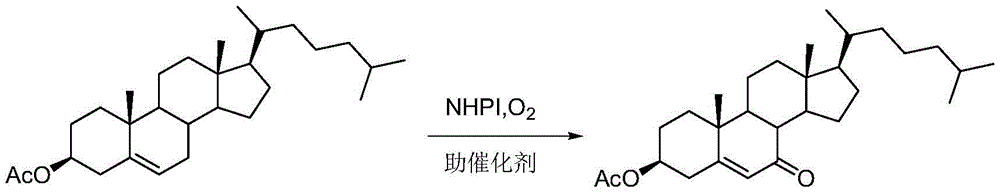
Synthesis method of vitamin D3 intermediate 7-ketocholesteryl acetate
A technique for the synthesis of cholesterol acetate, which is applied in the fields of steroids, chemical recovery, organic chemistry, etc., can solve the problems of complex post-processing, high cost, heavy metal environmental pollution, etc., and achieve high conversion rate and selectivity, and reaction Fast and gentle, fully decomposed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a self-priming reactor, put 200g (0.47mol) of cholesterol acetate, 22.84g (0.14mol) of NHPI, add 1000mL of heptane, 760mL of tetrahydrofuran, and heat to 65°C to dissolve. At this time, add 0.57g (0.0024mol) ) benzoyl peroxide, maintain a slight positive pressure in the system with oxygen during the whole reaction process, stir and reflux for 4h. After 4 hours, add 3.4 g (0.0094 mol) of cetyltrimethylammonium bromide, close the reflux pipe valve, and begin to distill out the aqueous solution of tetrahydrofuran. As tetrahydrofuran is continuously distilled out, tetrahydrofuran must be added dropwise to ensure The amount of tetrahydrofuran, keep the reaction for 2h, remove most of the tetrahydrofuran from the reaction solution under reduced pressure, and precipitate a large amount of solid, which is the catalyst NHPI, filter, and the catalyst can be used mechanically after being washed with heptane; 184.5 g of 7-ketocholesteryl acetate was obtained, the content of whic...
Embodiment 2
[0021] In a self-priming reactor, put 200g (0.47mol) of cholesterol acetate, 22.84g (0.14mol) of NHPI, add 1000mL of heptane, 760mL of tetrahydrofuran, and heat to 65°C to dissolve. At this time, add 0.57g (0.0024mol) ) benzoyl peroxide, maintain a slight positive pressure in the system with oxygen during the whole reaction process, stir and reflux for 4h. After 4 hours, add 3.0 g (0.0094 mol) of tetrabutylammonium bromide, close the reflux pipe valve, and begin to distill the aqueous solution of tetrahydrofuran. As tetrahydrofuran is continuously distilled out, tetrahydrofuran must be added dropwise to ensure the amount of tetrahydrofuran in the system. Keep the reaction for 2h, remove most of the tetrahydrofuran from the reaction solution under reduced pressure, and precipitate a large amount of solid, which is the catalyst NHPI, filter it, and wash the catalyst with heptane before applying it; remove the heptane from the filtrate under reduced pressure, add methanol to cryst...
Embodiment 3
[0023] In a self-priming reactor, put 200g (0.47mol) of cholesterol acetate, 38.07g (0.23mol) of NHPI, add 1000mL of heptane, 760mL of tetrahydrofuran, and heat to 65°C to dissolve. At this time, add 0.57g (0.0024mol) ) benzoyl peroxide, maintain a slight positive pressure in the system with oxygen during the whole reaction process, stir and reflux for 4h. After 4 hours, add 3.0 g (0.0094 mol) of tetrabutylammonium bromide, close the reflux pipe valve, and begin to distill the aqueous solution of tetrahydrofuran. As tetrahydrofuran is continuously distilled out, tetrahydrofuran must be added dropwise to ensure the amount of tetrahydrofuran in the system. Keep the reaction for 2h, remove most of the tetrahydrofuran from the reaction solution under reduced pressure, and precipitate a large amount of solid, which is the catalyst NHPI, filter it, and wash the catalyst with heptane before applying it; remove the heptane from the filtrate under reduced pressure, add methanol to cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com