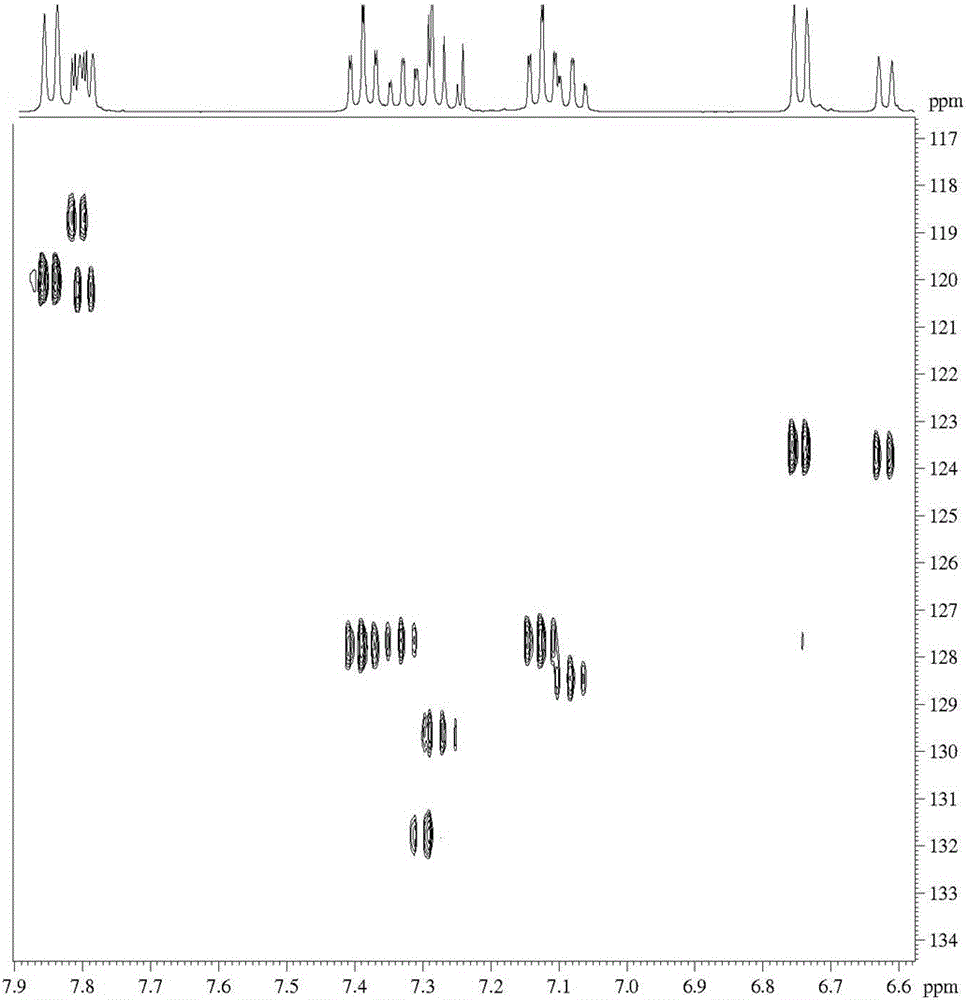
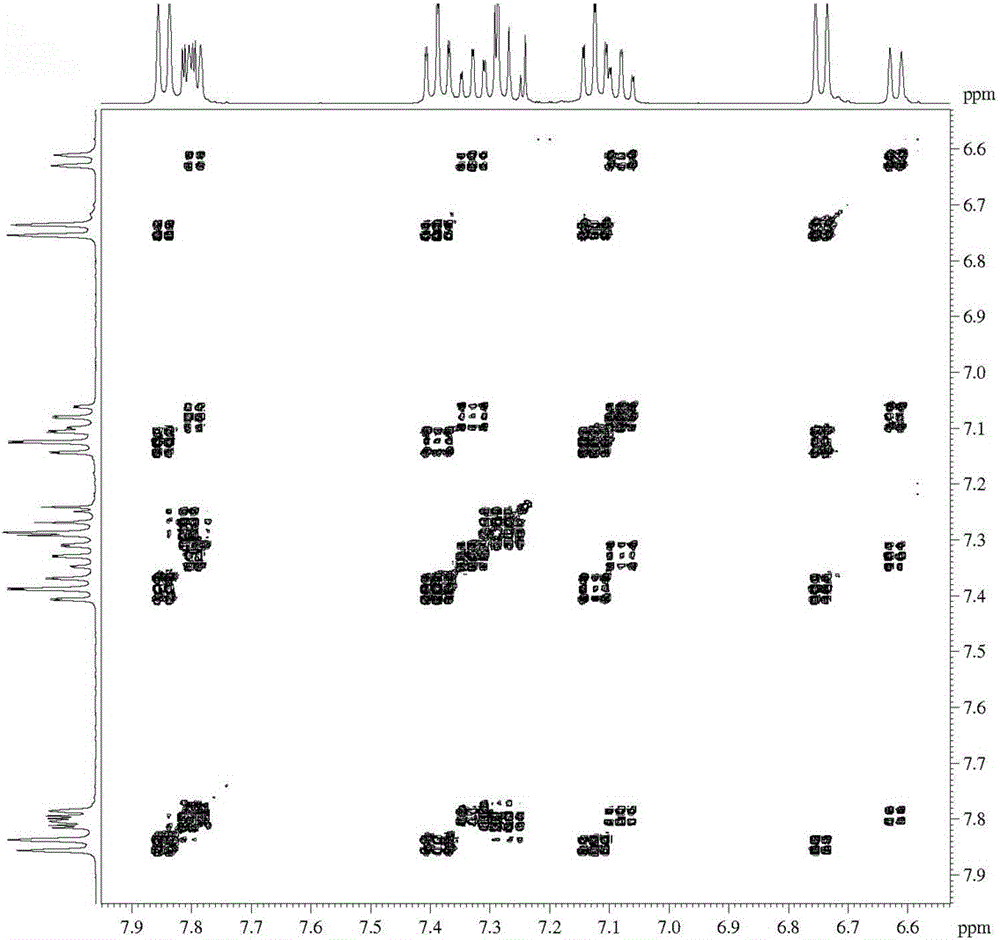
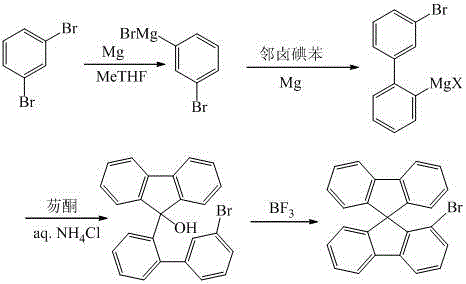
Synthesis method of 1-bromo-9,9'-spirodifluorene
A synthesis method, the technology of spirobifluorene, applied in the field of organic chemical synthesis, can solve the problems of 1-bromofluorenone, which is expensive, difficult to industrialize production, and affects the potential application of luminescent materials, etc., and achieves improved solubility, reduced cost, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022]
[0023] Under argon protection, 6.0 g (0.25 mol) of magnesium powder was added to a 500 mL three-neck flask, and immersed in 30 mL of methyltetrahydrofuran (dry) solution. Under stirring, add about 5mL of 1,3-dibromobenzene in methyl tetrahydrofuran dropwise to the above reaction solution, after the Grignard reagent triggers, slowly drop in the remaining 1,3-dibromobenzene in methyl tetrahydrofuran solution 100mL , add a total of 47.2g (0.2mol) 1,3-dibromobenzene, after dripping, reflux for 6h to synthesize 3-bromophenylmagnesium bromide; then under the protection of argon, first add 4.5g (0.02mol) of zinc bromide, Then add 100mL o-xylene solution containing 65.8g (0.2mol) of 1,2-diiodobenzene and 4.8g (0.2mol) of magnesium powder to the above reaction solution, control the reaction temperature at 90°C, and reflux for 10h to form 3-Bromobiphenyl magnesium iodide. Under the protection of argon, add 36 g (0.2 mol) of fluorenone in methyl tetrahydrofuran solution drop...
example 2
[0027]
[0028] Under argon protection, 18.0 g (0.75 mol) of magnesium powder was added to a 1 L three-necked flask, and immersed in 100 mL of methyl tetrahydrofuran (dry) solution. Under stirring, add about 5mL of 1,3-dibromobenzene in methyl tetrahydrofuran dropwise to the above reaction solution, after the Grignard reagent triggers, slowly drop in the remaining 1,3-dibromobenzene in methyl tetrahydrofuran solution 200mL , add 165.2g (0.7mol) 1,3-dibromobenzene in total, after dropping, reflux for 7h to synthesize 3-bromophenylmagnesium bromide; then under the protection of argon, first add 7.12g (0.032mol) 300mL of o-xylene solution containing 188.1g (0.665mol) of o-bromoiodobenzene was dropped into the above reaction solution, the reaction temperature was controlled at 120°C, and the reaction was refluxed for 12 hours to generate 3-bromobiphenylmagnesium bromide. Under the protection of argon, add 113.5 g (0.63 mol) of fluorenone in methyl tetrahydrofuran solution dropw...
example 3
[0030]
[0031]Under the protection of argon, put 10.8g (0.45mol) of magnesium powder into a 1L three-necked flask, and immerse it with 100mL of methyltetrahydrofuran (dry) solution. Under stirring, add about 5mL of 1,3-dibromobenzene in methyl tetrahydrofuran dropwise to the above reaction solution, after the Grignard reagent triggers, slowly drop in the remaining 1,3-dibromobenzene in methyl tetrahydrofuran 300mL , a total of 94.4g (0.4mol) of 1,3-dibromobenzene was added, after dropping, refluxed for 6h to synthesize 3-bromophenylmagnesium bromide; then under the protection of argon, 19.8g (0.088mol) of zinc bromide was first added, Then 200mL of o-xylene solution containing 104.9g (0.44mol) of o-chloroiodobenzene was dropped into the above reaction solution, the reaction temperature was controlled at 140°C, and the reaction was refluxed for 8 hours to generate 3-bromobiphenylmagnesium chloride. Under the protection of argon, add 72g (0.4mol) of fluorenone in methyl tetr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com