Genistein derivative as well as preparation method and application thereof in pharmacy
A technology of genistein and derivatives, applied in the field of medicine, can solve the problem that the anti-tumor effect is not fully elucidated, and achieve the effect of good inhibition of tumor cell invasion and migration, and good therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of genistein derivatives: genistein 2.7g (10.0mmol), dehydrated alcohol 150mL, heat to dissolve, add potassium carbonate 1.5g (11.0mmol), potassium iodide catalytic amount, epichlorohydrin 9.3g ( 0.10mol), refluxed for 10h, filtered while it was hot, cooled, precipitated crystals, and filtered with suction to obtain 1.64g of a light yellow solid with a yield of 42.9%, which was 7,4'-bis(2,3-epoxypropoxy) -5-Hydroxyisoflavone, 7,4'-bis(2,3-epoxypropoxy)-5-hydroxyisoflavone is directly put into the next step reaction; 7,4'-bis(2,3-epoxypropyl Oxygen)-5-hydroxyisoflavone 500mg (1.3mmol), absolute ethanol 30mL, heat to dissolve, add piperidine 340mg (4.0mmol), reflux for 5h, product column chromatography (chloroform:methanol=100:3), 135 mg of pale white solid (compound GEN-27) was obtained, with a yield of 18.7%.
[0048] Physicochemical properties of compound GEN-27: M.p.140~143℃; ESI-MSm / z:553[M+H] + , suggesting a molecular weight of 552 and a mo...
Embodiment 2
[0052] 1. Experimental materials
[0053] (1) Drugs
[0054] Genistein derivatives (GEN-27), purity>99.5%; genistein (GEN), purity>99.5%.
[0055] GEN-27 and GEN were made into 100mM stock solutions with DMSO, stored at -20°C, and sample solutions with required concentrations were made with RPMI-1640 medium containing 10% fetal bovine serum before use.
[0056] (2) cell line
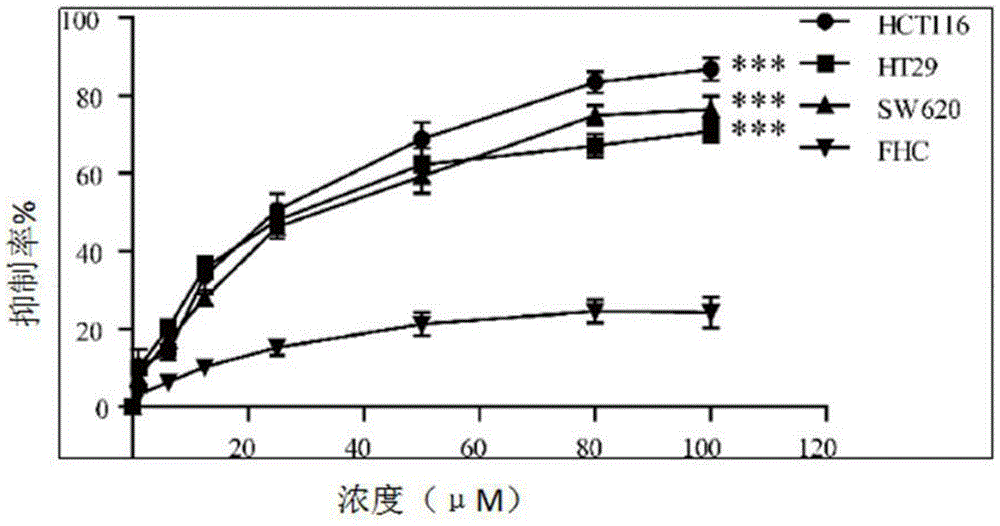
[0057] Human colon cancer HCT116 cell line, HT29 cell line, SW620 cell line, human normal colonic mucosal epithelial cell FHC cell line were from the cell bank of the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, with 10% fetal bovine serum Cultured in RPMI-1640 medium (the same below).
[0058] (3) Reagents
[0059] 1) RPMI-1640 medium: Dissolve 10.4 g of RPMI-1640 powder (product of GIBCO, USA) in 1000 mL of sterilized three-distilled water, and use NaHCO 3 Adjust the pH value to 7.3-7.4, filter and sterilize with a cylindrica...
Embodiment 3
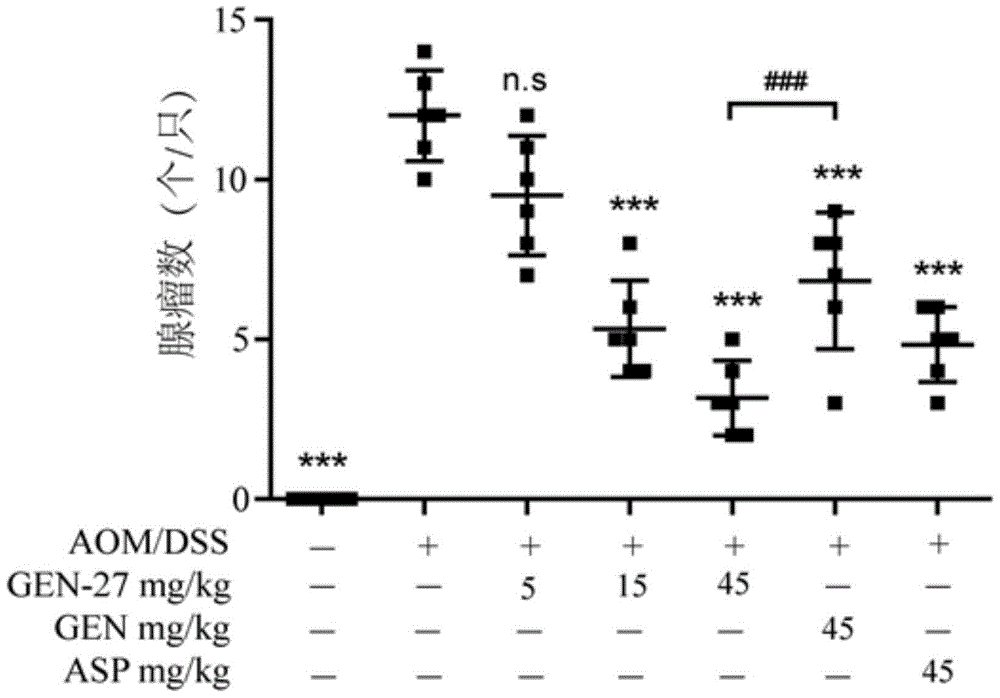
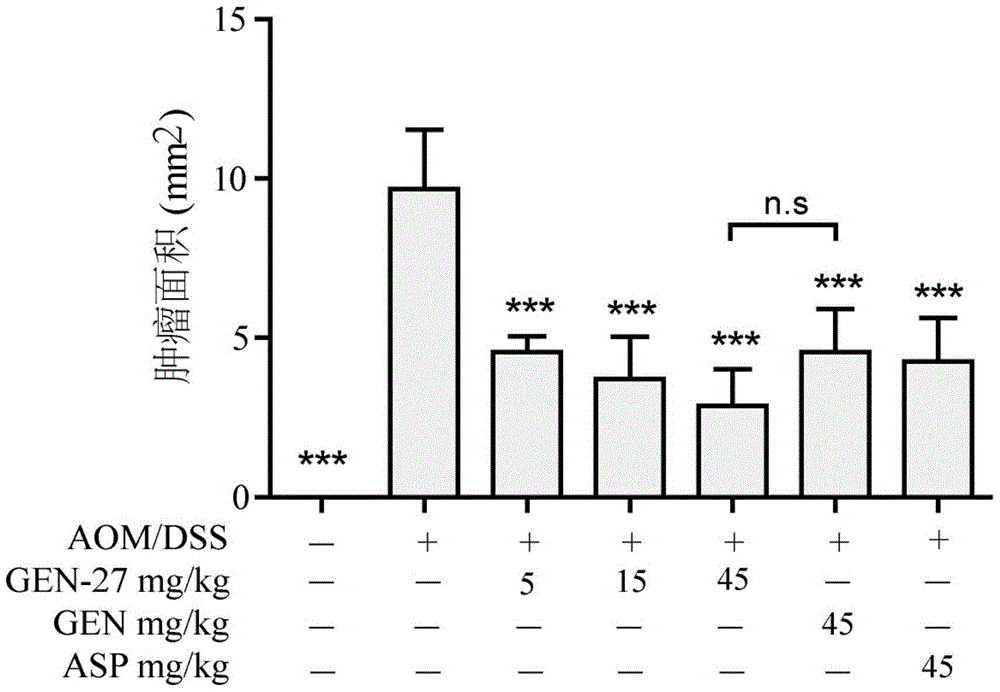
[0082] To investigate the preventive effect of compound GEN-27 on the azoxymethane (AOM) / dextran sodium sulfate (dextransodiumsulfate, DSS)-induced ulcerative colitis-associated colorectal cancer model in mice.
[0083] Administration method: the test compound was mixed into the mouse food according to the corresponding dose concentration, and administered by feeding method.
[0084]Establishment of AOM / DSS mouse colorectal cancer model: C57BL / 6 mice (purchased from Nanjing Institute of Biomedicine, Nanjing University), 70 healthy, vigorous male mice weighing 18-20 g were randomly divided into 7 Group, 10 in each group. Seven days after the mice adapted to a normal diet, it was recorded as the start day of the experiment, and the normal diet was replaced with a diet containing the corresponding concentration of the compound. On the 14th day after the start of the experiment, each mouse was intraperitoneally injected (i.p.) 10.0 mg / kg of azoxymethane, and after 7 days, it was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com