Wolfberry glutathione reductase and encoding gene and application
A technology of glutathione and coding genes, applied in oxidoreductase, application, genetic engineering, etc., can solve the problems of high radiation hazard, heavy screening workload, heavy breeding work, etc., and achieve high tolerance to cadmium ions, The effect of high salt tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Cloning of Glutathione Reductase LcGR Gene from Lycium barbarum
[0030] Utilize Trizol reagent, extract total RNA from 100mg of fresh Lycium barbarum (Chinese wolfberry) leaves, use Transgentransecriptone-stepgDNAremovalandcDNAsynthesissupermix kit, take Lycium barbarum total RNA as template, OligodT 18 As a primer, the first strand of cDNA was synthesized under the action of AMV reverse transcriptase.
[0031] According to the Unigene sequence design of Lycium barbarum transcriptome database
[0032] The upstream primer LcGRW-F shown in SEQIDNO.3: 5'TGGAAAGCCTACTAAAGCCTGAC3' and the downstream primer LcGRW-R shown in SEQIDNO.4: 5'CAACATTTCTCTATCGGCCCTC3',
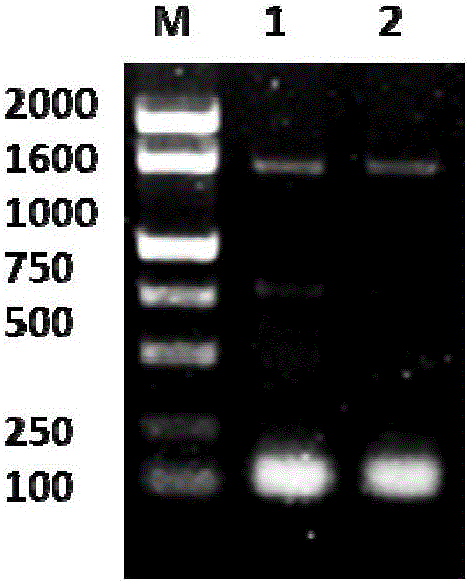
[0033] The tail fragment of the glutathione reductase LcGR gene in Lycium barbarum was amplified by PCR, and the electrophoresis of the PCR product is shown in Figure 1(a) after sequencing verification. Tail primer P2 was designed. Design upstream primers according to the Unigene sequence of Lycium barbarum tran...
Embodiment 2
[0039] Construction process of recombinant vector pMD18-T-LcGR
[0040] The LcGR gene shown in the sequence listing was connected to the pMD18-T vector,
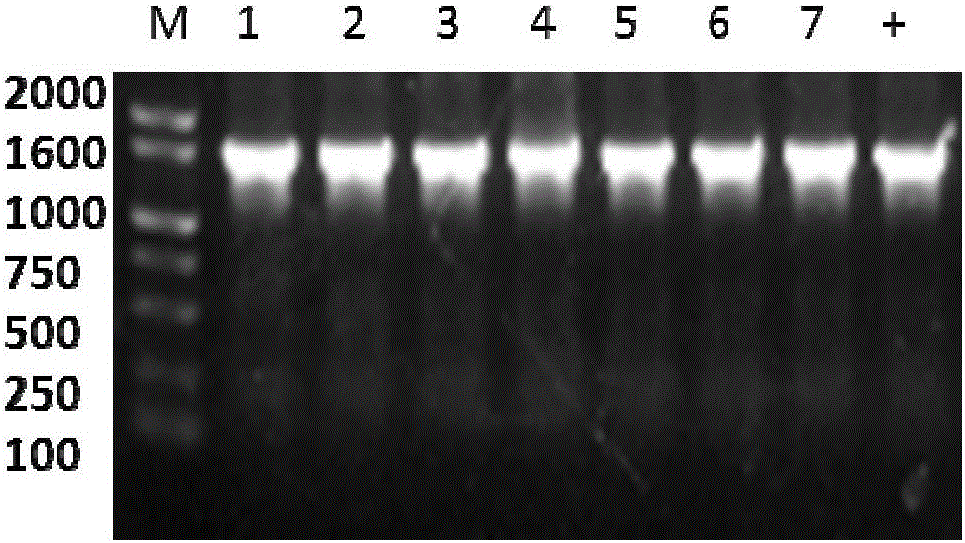
[0041] Reaction conditions: 16°C, 30min. The ligation product was transformed into E.ColiTop10. Pick white colonies, colony PCR method to confirm the length of the insert in the T vector, such as figure 2 , as expected, the vector was sent to Huada Gene Company for sequencing, and we obtained the 1609bp deoxynucleotide sequence of the gene, which was blasted at NCBI and showed high homology with tomato, potato, etc., indicating that the gene was cloned successfully . The LcGR deoxynucleotide sequence and the pMD18-T sequence were spliced and assembled into the cloning vector pMD18-T-LcGR, such as image 3 shown
Embodiment 3
[0043] Construction process of prokaryotic recombinant expression vector pET28a-LcGR in Escherichia coli
[0044] First, the pMD18-T-LcGR plasmid was used as a template,
[0045] P3 shown in SEQ ID NO.7 (P3:CGC GGATCC ATGGCTAGGAAGATGTTGATTG),
[0046] P4 shown in SEQ ID NO.8 (P4:ACGC GTC GAC ACTCTCGTCACTCCATTTTCGT) are upstream and downstream primers, respectively, to amplify the LcGR gene. The reaction conditions are: 94°C, 4min; (94°C, 30Sec; 56°C, 30Sec; 72°C, 1min50Sec) 32cycles; 72°C, 8min.
[0047] There is a BamHI restriction site (GGATCC) in P3 and a SalI restriction site (GTCGAC) in P4, then the PCR product and the pET28a empty vector plasmid are double-digested with BamHI and SalI respectively, and the digested products of the two are Ligation, the ligation product was transformed into E.ColiDH5α, spread on LB plates containing 200 mg / L kana resistance, and cultured at 37°C. After 12 hours, pick a single colony for colony PCR verification, such as Figure 4 ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com