Method for directly preparing molten steel from high-silicon iron ores
A direct, iron ore technology, applied in the field of direct preparation of molten steel from high-silicon iron ore, can solve the problems of inability to obtain low-silicon molten iron, rising beneficiation costs, and high iron loss, avoiding iron-smelting and steel-making processes and reducing refining steps , the effect of short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
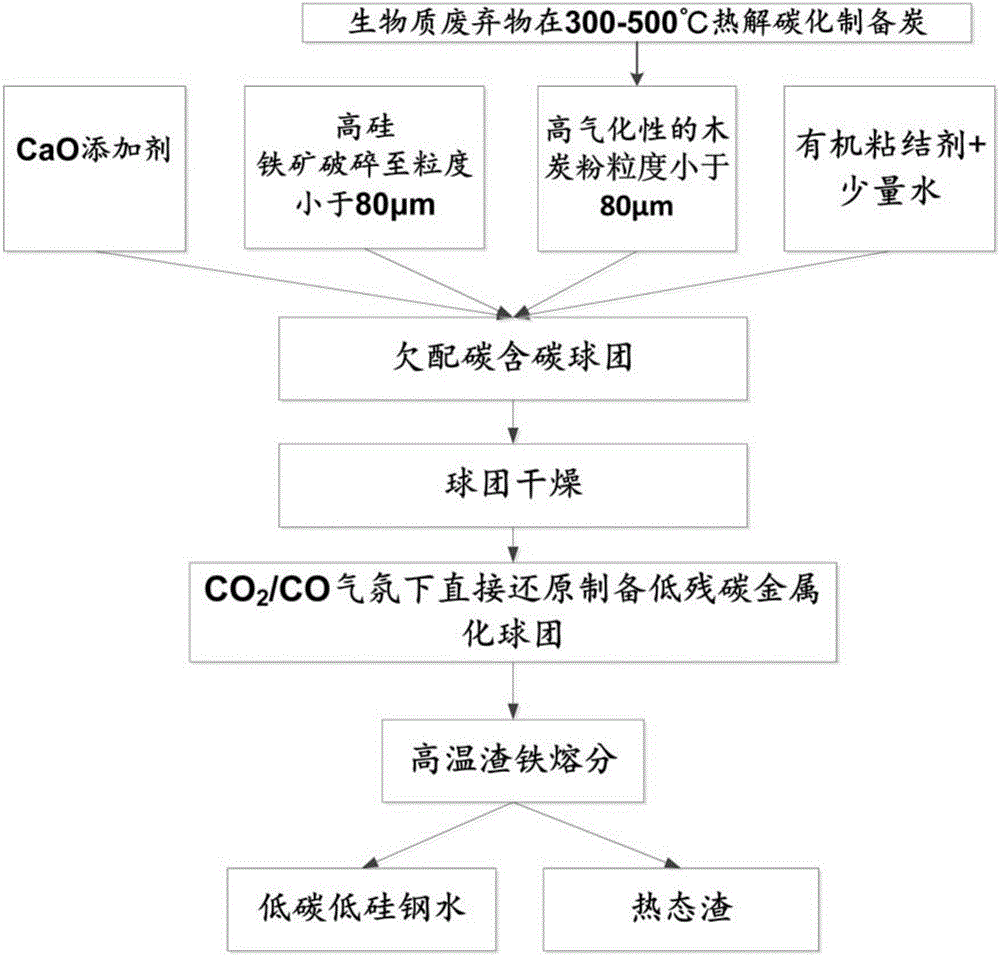
[0049] Step 1. Preparation of biochar: Waste biomass is pyrolyzed under the condition of 300-500° C. in isolation from air to produce biochar, and the obtained biochar is ball-milled into fine-grained charcoal powder.
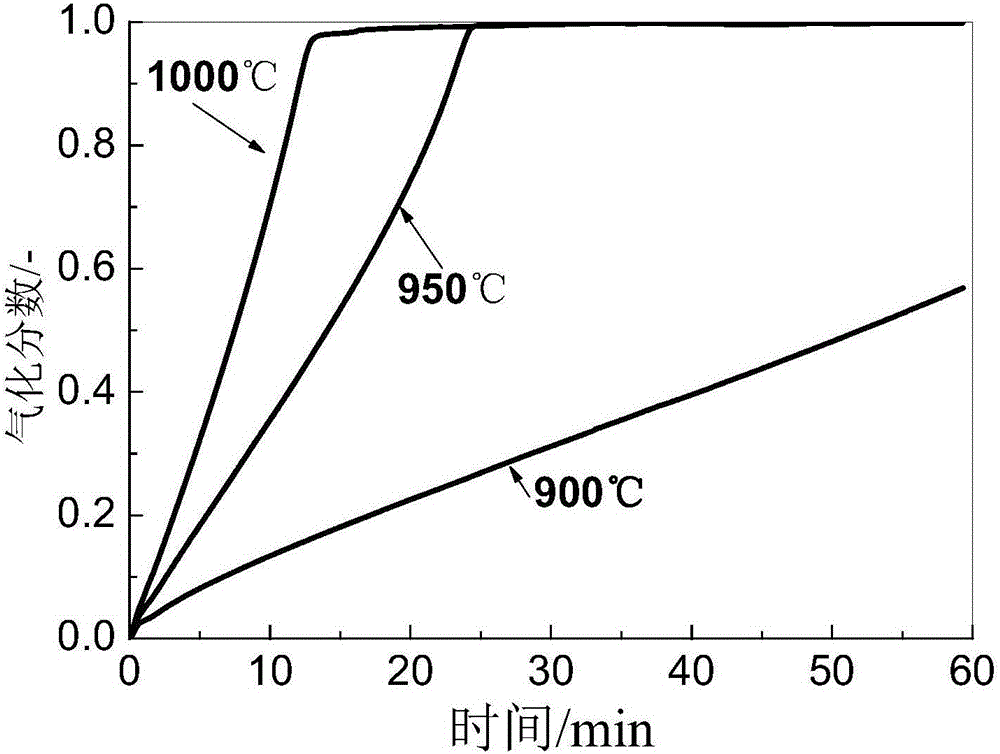
[0050] The fixed carbon content in the biomass charcoal obtained in this step is greater than 60wt%, the volatile matter content is greater than 30wt%, and the ash content is less than 5wt%. After crushing and ball milling, the particle size of the fine-grained biomass charcoal powder is less than 80 μm. at 1000°C and CO 2 The partial pressure is greater than 0.5atm, and the biomass charcoal powder can be completely gasified within 10 minutes.
[0051] Step 2, ore crushing: take high silicon iron ore as raw material, the total iron content of the high silicon iron ore is greater than 55wt%, and the silicon dioxide content is greater than 10wt%, and it is broken into fine granular mineral powder with a crusher and a ball mill , The particle size of mineral powd...
Embodiment 1
[0066][1]. Preparation of biochar: waste jujube wood was pyrolyzed and carbonized at 400℃ to prepare biochar. The industrial analysis results of the obtained biochar are shown in Table 2. The gasification performance of the obtained biochar is as follows: figure 2 shown. The prepared biochar is ball-milled, and its particle size is less than 80 μm.
[0067] Table 2. The industrial analysis result (wt%) of biochar used in embodiment one
[0068] .
[0069] [2]. Mineral material crushing: take 500g of the above-mentioned high silicon iron ore, preliminarily crush and fully ball mill, and its particle size will reach less than 80μm.
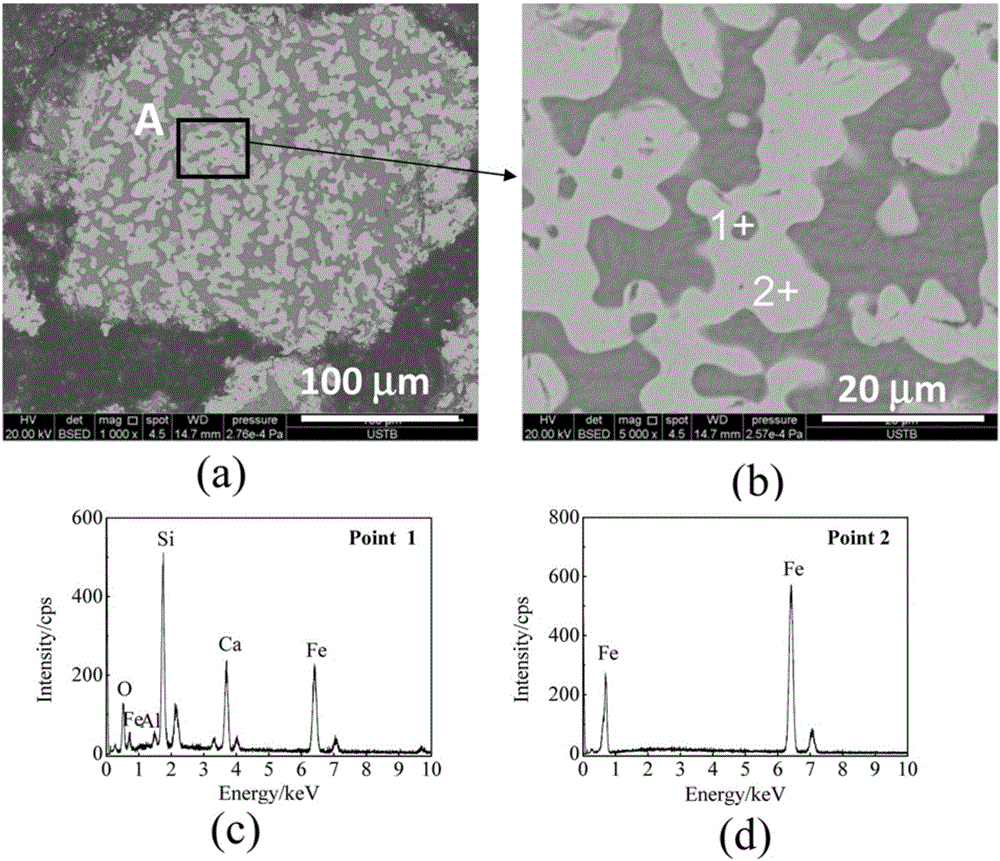
[0070] [3]. Pellet preparation: mix the above-mentioned iron ore powder, charcoal powder and a certain amount of CaO. The amount of biochar used is 122g, that is, the amount satisfies that the molar ratio of C / O in the carbon-containing pellets is 0.8. Wherein CaO addition satisfies CaOwt% / SiO 2 wt% = 2.0. The mixed material was made into...
Embodiment 2
[0077] [1]. Preparation of biochar: same as Example 1.
[0078] [2]. Mineral material crushing: take 500g of the above-mentioned high silicon iron ore, preliminarily crush and fully ball mill, and its particle size will reach less than 80μm.
[0079] [3]. Pellet preparation: mix the above-mentioned iron ore powder, charcoal powder and a certain amount of CaO. The amount of biochar used is 122g, that is, the amount satisfies that the molar ratio of C / O in the carbon-containing pellets is 0.8. Wherein CaO addition satisfies CaOwt% / SiO 2 wt% = 1.0. The mixed material was made into pellets with a diameter of 10.0 mm. The ball binder uses 2.0% waste paper pulp.
[0080] [4]. Pellet drying: After the pellets were dried naturally for 24 hours, they were further dried at 300°C for 2 hours.
[0081] [5]. Direct reduction: Reduction under the following conditions: use a tube furnace, the reduction temperature is 1100 ° C, the reduction time is 20 minutes, and CO / CO is introduced 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com