Acid- and radical-generating agent and method for generating acid and radical
A technology of carbonyloxy and carbonylamino, which is applied in the direction of chemical instruments and methods, photographic plate-making process of patterned surface, photosensitive material used in optomechanical equipment, etc., can solve the problems of poor stability, dimensional change, and easy resist performance. Changes and other problems, to achieve the effect of heat resistance, good efficiency, high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0412] Hereinafter, the present invention will be specifically described based on examples and comparative examples. However, the present invention is not limited by these examples.
Synthetic example 12
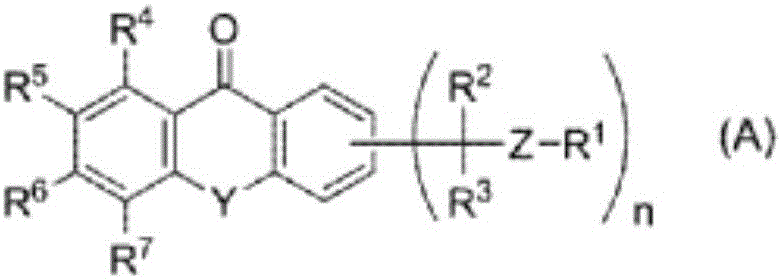
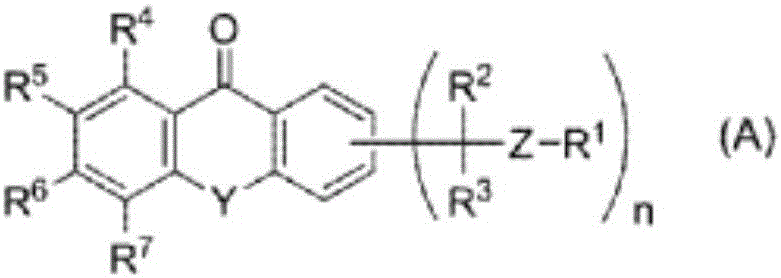
[0413] Synthesis Example 12, Synthesis of 4-bis(1-bromoethyl)thioxanth-9-one
[0414] 26.8 g (100 mmol; manufactured by Wako Pure Chemical Industries, Ltd.) of 2,4-diethylthioxanth-9-one was dissolved in 300 mL of ethyl acetate, and N-bromosuccinimide 19.6 g (110mmol; manufactured by Wako Pure Chemical Industries, Ltd.) and 2,2'-azobis(4-methoxy-2,4-dimethylvaleronitrile) 1.54g (5.0mmol; Wako Pure Chemical Industries ( Co., Ltd.), stirred at 45°C for 30 minutes. Next, the reaction liquid was temporarily cooled to room temperature, and then, 19.6 g (110 mmol; manufactured by Wako Pure Chemical Industries, Ltd.) of N-bromosuccinimide and 2,2'-azobis( 4-methoxy-2,4-dimethylvaleronitrile) 1.54 g (5.0 mmol; manufactured by Wako Pure Chemical Industries, Ltd.), and stirred at 45° C. for 1 hour. After the reaction was completed, methanol was added to the reaction liquid, cooled to 5° C., and the obtained crystals were deliquified, thereby obtaining 28.9 g (micro Yellow powder, yie...
Embodiment 12
[0417] Example 12, Synthesis of 4-bis(1-tosylethyl)thioxanth-9-one (compound represented by formula (1))
[0418] 4.26 g (10.0 mmol; manufactured by Wako Pure Chemical Industries, Ltd.) of 2,4-bis(1-bromoethyl)thioxanth-9-one obtained in Synthesis Example 1 and p-sodium toluenesulfinate 5.0 g (20.0 mmol; manufactured by Wako Pure Chemical Industries, Ltd.) of 4 water mixtures was dissolved in 20 mL of dimethylformamide (DMF), and stirred at room temperature for 1 hour. After the reaction was completed, water was added to the reaction solution, and the precipitate generated by adding water was obtained by filtration, and the obtained filtrate was recrystallized in dioxane, thereby obtaining 2,4-bis(1-toluenesulfonate Acylethyl)thioxanth-9-one 0.84 g (light yellow powder, yield: 19%). Below, the 1 H-NMR measurement results and structural formula of 2,4-bis(1-tosylethyl)thioxanth-9-one.
[0419] 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 1.63 (3H, d), 1.82 (3H, d), 2.28 (3H, s), 2.38...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com